含铜铁液与熔渣界面上Cu迁移的极化效应
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
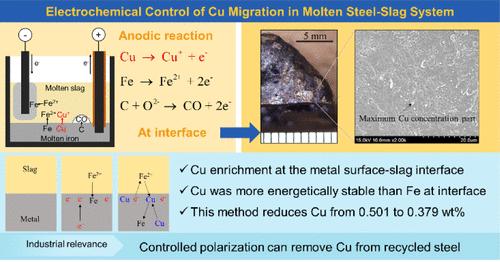
在炼钢行业,使用以废钢为原料的电弧炉是最有希望显著减少二氧化碳排放的方法。然而,铁水中的铜是导致钢铁冶金和机械问题的主要污染物。在本研究中,我们通过实验探索了在1823 K下恒压电解从含铜铁液中分离铜的可行性。当工业熔渣中FeO含量为25.5 wt %, S含量为0.05 wt %时,阳极极化下的脱铜速率随电压升高而降低,而阴极极化下的脱铜速率则升高。在- 10 V的Ar-5 vol % O2气氛中,金属相中的Cu浓度从0.501 wt %下降到0.379 wt %。分子动力学模拟显示,在渣-金属界面处存在富Cu相,Cu在该界面处比Fe更能量稳定。金属中的Cu由于具有较高的电子亲和性,往往带负电荷,在过量电荷的情况下,将炉渣中的Cu原子吸引到界面上。因此,阳极极化对工业渣电精炼脱铜无效,而阴极极化使界面处的Cu浓度升高。铜浓度可以通过施加电位来控制,这在产生最终产品的过程中是有用的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Polarization Effects on Cu Migration at the Interface between Cu-Containing Molten Fe and Molten Slag
In the steelmaking industry, using electric arc furnaces with scrap as raw material is the most promising method to significantly reduce CO2 emissions. However, Cu in molten iron is a major contaminant that causes metallurgical and mechanical issues in steel. In this study, we experimentally explored the feasibility of separating Cu from molten Cu-containing iron by constant-voltage electrolysis at 1823 K using molten slag from actual processes. With industrial molten slag containing 25.5 wt % FeO and 0.05 wt % S, the decopperization rate decreased with increasing voltage under anode polarization, whereas it increased under cathodic polarization. The Cu concentration in the metal phase dropped from 0.501 to 0.379 wt % at −10 V in an Ar-5 vol % O2 atmosphere. Molecular dynamics simulations revealed a Cu-rich phase at the slag–metal interface, with Cu being more energetically stable than Fe at this boundary. Cu in the metal tended to be negatively charged owing to its high electron affinity, attracting Cu atoms in the slag to the interface under excess charge. Thus, anodic polarization is ineffective for decoppering in industrial slag electrorefining, whereas cathodic polarization increases Cu concentration at the interface. The Cu concentration can be controlled by applying a potential, which is useful in processes leading to the final product.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


