六氰高铁酸镍泡沫电极的吸附选择性及影响因素:从伪海水中提取98%的钾组分溶液
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
金属六氰高铁酸盐具有通过氧化还原反应选择性阳离子插入到其三维晶格中的能力,是一种很有前途的海水和废水脱盐和浓缩吸附剂。从溶液中去除阳离子的分数量之间的比率是通常用于评价吸附选择性的度量。然而,这一指标也直接取决于吸附溶液中的阳离子浓度,从而通过反应产率取决于电极电位。在这里,我们分析了具有高孔隙率和优异离子扩散性的NiHCF泡沫电极的吸附选择性,仅使用离子交换的选择系数,即独立于电极电位。我们进行了一个钾提取实验,有三个连续的阶段,每个阶段包括首先吸附和解吸过程。以伪海水(K+ = 10 mmol/L, Na+ = 495 mmol/L)为第一吸附溶液,得到高钾分数(98%;K+ = 123 mmol/L, Na+ = 3 mmol/L)。时间浓度变化表明,测量值与仅使用离子交换选择系数计算的值之间非常吻合,表明NiHCF泡沫电极的吸附选择性主要受离子交换反应的影响,而不依赖于电极电位。我们还通过循环性评估和详细的K+吸附选择性评估证明了泡沫电极在工业应用中的实用性,合成海水是一种更现实的海水模拟物,不仅含有Na+和K+,还含有二价阳离子(Mg2+, Ca2+)。本文章由计算机程序翻译,如有差异,请以英文原文为准。

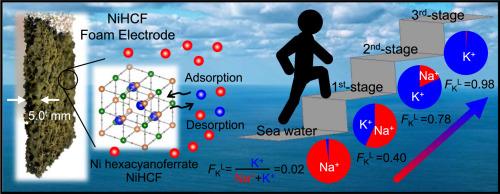
Adsorption selectivity of nickel hexacyanoferrate foam electrodes and influencing factors: extraction of a 98 % potassium fraction solution from pseudo-seawater
Metal hexacyanoferrates are promising adsorbents for desalination and concentration of seawater and wastewater, because of a high capacity for selective cation intercalation into their three-dimensional lattice through redox reactions. The ratio between fractional quantities of cations removed from a solution is a metric commonly used to evaluate adsorption selectivity. However, this metric also depends directly on cation concentrations in the adsorption solution, thus on the electrode potential through the reaction yield. Here, we analyzed the adsorption selectivity of nickel hexacyanoferrate (NiHCF) foam electrodes, characterized by high porosity and excellent ion diffusivity, using only the selectivity coefficient for ion exchange, i.e., independently of the electrode potential. We conducted a potassium extraction experiment with three consecutive stages, each comprising first an adsorption then a desorption process. From pseudo-seawater (K+ = 10 mmol/L, Na+ = 495 mmol/L) as first adsorption solution, we obtained a final desorption solution with a high potassium fraction (98 %; K+ = 123 mmol/L, Na+ = 3 mmol/L). Temporal concentration variations illustrated the close agreement between measurements and values calculated using only the selectivity coefficient for ion exchange, demonstrating that the adsorption selectivity of NiHCF foam electrodes was primarily influenced by ion exchange reactions, and did not depend on the electrode potential. We also demonstrated the usefulness of our foam electrodes for industrial application through a cyclability assessment and a detailed K+ adsorption selectivity evaluation in synthetic seawater, a more realistic seawater analogue containing not only Na+ and K+, but also divalent cations (Mg2+, Ca2+).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


