巨噬细胞在缺血再灌注损伤中的动态调控及靶向干预
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
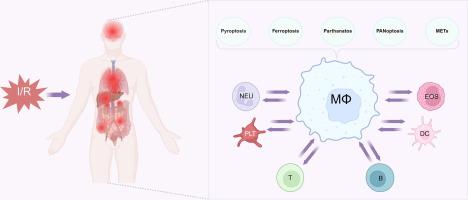
缺血再灌注损伤(IRI)是一个复杂的病理生理过程,以组织再灌注过程中的氧化应激和炎症反应为特征,导致严重的器官功能障碍。巨噬细胞作为关键的免疫细胞,在IRI的发病机制中起关键作用,表现出影响组织损伤和修复的动态功能。尽管进行了广泛的研究,但巨噬细胞介导的IRI的确切机制仍不完全清楚,因此有必要对其多方面的作用和潜在的治疗靶点进行全面的综述。综述目的:本文旨在阐明巨噬细胞在IRI中的多种作用,重点阐述巨噬细胞参与细胞程序性死亡机制、与其他免疫细胞的通讯以及对IRI影响的关键器官的调节作用。该综述还探讨了针对巨噬细胞的潜在治疗策略,以减轻iri诱导的损伤。本文综述了巨噬细胞在IRI中的多重作用,并探讨了IRI诱导的巨噬细胞程序性死亡的各种模式,包括气凝胶蛋白d介导的焦亡,脂质过氧化相关的铁亡,parp -1介导的par依赖性细胞死亡,PANoptosome调节的PANoptosis,以及活性氧依赖和非依赖途径诱导的巨噬细胞胞外陷阱(METs)的形成。此外,本文还讨论了IRI中巨噬细胞与其他免疫细胞之间的细胞间通讯,重点讨论了巨噬细胞与中性粒细胞之间的双向调节作用,以及它们在解决炎症中的协同作用。此外,系统总结了巨噬细胞在IRI中影响脑、肺、心、肾、肝等关键器官的调控机制。最后,深入分析了针对巨噬细胞的创新治疗策略,包括调节细胞极化、抑制过度METs形成和利用纳米药物递送系统等精确方法。本综述为IRI的临床转化研究提供了重要的理论基础。缺血再灌注损伤(ischemia - reperfusion Injury, IRI)是指缺血一段时间后血流恢复后所发生的功能和结构改变。IRI不仅是许多疾病病理进展的关键因素,而且有助于延迟移植物恢复。尽管IRI在各种器官中的作用已被广泛研究,但其确切的机制和途径仍然知之甚少,并且存在很大争议。除缺血外,再灌注本身可加剧组织和器官损伤,特别是通过炎症过程。在正常情况下,巨噬细胞保护机体免受感染并调节组织炎症。在缺血过程中,巨噬细胞被多种信号激活,通过释放活性氧(ROS)、促炎细胞因子和趋化因子引发炎症反应。再灌注时,巨噬细胞聚集在损伤部位,在那里它们发挥双重功能。一方面,它们通过产生细胞因子加重炎症和氧化应激,从而加重组织损伤和功能障碍[1,2]。另一方面,它们通过清除细胞碎片、促进血管生成和释放支持组织愈合的生长因子来促进组织修复和再生[3,4]。因此,全面了解IRI中巨噬细胞介导的组织损伤和修复的分子机制,并制定有针对性的治疗策略,对于IRI的临床预防和治疗至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dynamic regulation and targeted interventions of macrophages in ischemia–reperfusion injury
Background
Ischemia-Reperfusion Injury (IRI) is a complex pathophysiological process characterized by oxidative stress and inflammatory responses during tissue reperfusion, leading to severe organ dysfunction. Macrophages, as key immune cells, play a pivotal role in the pathogenesis of IRI, exhibiting dynamic functions that influence both tissue damage and repair. Despite extensive research, the precise mechanisms underlying macrophage-mediated IRI remain incompletely understood, necessitating a comprehensive review to explore their multifaceted roles and potential therapeutic targets.Aim of Review: This review aims to elucidate the diverse roles of macrophages in IRI, focusing on their involvement in programmed cell death mechanisms, communication with other immune cells, and regulatory effects on key organs affected by IRI. The review also explores potential therapeutic strategies targeting macrophages to mitigate IRI-induced injury.Key Scientific Concepts of Review: This article reviews the multifaceted roles of macrophages in IRI and explores various modes of macrophage programmed cell death induced by IRI, including gasdermin D-mediated pyroptosis, lipid peroxidation-associated ferroptosis, PARP-1-mediated PAR-dependent cell death, PANoptosis regulated by the PANoptosome, and the formation of macrophage extracellular traps (METs) induced by both reactive oxygen species-dependent and −independent pathways. Additionally, it discusses intercellular communication between macrophages and other immune cells in IRI, focusing on the bidirectional regulatory effects between macrophages and neutrophils, as well as their synergistic role in resolving inflammation. Moreover, the regulatory mechanisms of macrophages in IRI affecting key organs, such as the brain, lung, heart, kidneys and liver, have been systematically summarized. Finally, innovative therapeutic strategies targeting macrophages, including precise approaches such as regulating cell polarization, inhibiting excessive METs formation, and utilizing nano-drug delivery systems, are thoroughly analyzed. This review provides a significant theoretical foundation for clinical translational research on IRI.Ischemia-Reperfusion Injury (IRI) refers to the functional and structural alterations that occur when blood flow is restored following a period of ischemia. IRI is not only a key factor in the pathological progression of many diseases but also contributes to delayed graft recovery. Although the role of IRI has been extensively studied in various organs, the precise mechanisms and pathways involved remain poorly understood and are highly contentious. Beyond ischemia, reperfusion itself can exacerbate tissue and organ damage, particularly through inflammatory processes. Under normal conditions, macrophages protect the body from infection and regulate tissue inflammation. During ischemia, macrophages are activated by diverse signals and initiate an inflammatory response by releasing oxygen species (ROS), pro-inflammatory cytokines, and chemokines. Upon reperfusion, macrophages accumulate at the injury site, where they exert a dual function. On the one hand, they exacerbate inflammation and oxidative stress by producing cytokines, thereby aggravating tissue damage and dysfunction [1,2]. On the other hand, they facilitate tissue repair and regeneration by clearing cellular debris, promoting angiogenesis, and releasing growth factors that support tissue healing [3,4]. Therefore, gaining a comprehensive understanding of the molecular mechanisms underlying macrophage-mediated tissue damage and repair during IRI, as well as developing targeted therapeutic strategies, is essential for the clinical prevention and treatment of IRI.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


