C(sp3) -N键在脱胺转化中裂解的自由基方法
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
胺是一种用途广泛的官能团,是有机合成的关键组成部分,特别是在药物开发中。胺的脱胺功能化,其中胺的C-N键被劈开作为反应的碳源,可能对构建有机分子的碳框架非常有用。传统的C(sp3) -N键裂解方法通常依赖于双电子离子途径或过渡金属催化,这通常需要活性胺或有毒试剂,限制了它们的广泛适用性。相比之下,基于自由基的方法提供了一种很有前途的替代方法,利用均溶C-N键裂解产生可以参与一系列转化的碳中心自由基。这些方法提供了互补的底物范围和独特的反应性,扩大了胺的合成效用。本文综述了自由基介导的脱胺转化的最新进展,重点介绍了机制、底物相容性以及在合成有机化学中的新应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
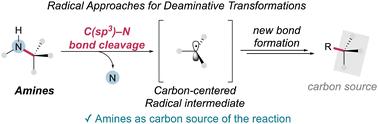
Radical approaches for C(sp3)–N bond cleavage in deaminative transformations
Amines are versatile and widely available functional groups that serve as key building blocks in organic synthesis, particularly in pharmaceutical development. The deaminative functionalization of amines, where the C–N bond of amine is cleaved to function as a carbon source of the reaction, could be very useful for building the carbon framework of organic molecules. Conventional methods for C(sp3)–N bond cleavage often rely on two-electron ionic pathways or transition metal catalysis, which typically require activated amines or toxic reagents, limiting their broad applicability. In contrast, radical-based approaches provide a promising alternative, utilizing homolytic C–N bond cleavage to generate carbon-centered radicals that can participate in a range of transformations. These methods offer complementary substrate scopes and unique reactivity profiles, expanding the synthetic utility of amines. This review summarizes recent advancements in radical-mediated deaminative transformations, focusing on the mechanisms, substrate compatibility, and emerging applications in synthetic organic chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


