硝酸和二氧化碳合成尿素一体化催化对的理论指导设计
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
二氧化碳和硝酸盐的电化学共还原为温室气体和含氮废弃物合成增值尿素提供了一条可持续的途径;然而,在设计高效催化剂方面仍然存在挑战。基于“整合催化对(icp)”的概念,设计了一种尿素合成催化剂,将杂原子(B和C)引入M-N-C中,其中单一过渡金属分散在n掺杂碳材料上。采用两步理论筛选策略,Ni - n3b具有较低的极限电位(- 0.43 V)和较小的C-N偶联动力学势垒(0.74 eV),这是由于Ni和B的电子调节作用以及Ni和B的协同作用增强NO3 -活化,促进气态CO2与*NH中间体之间的C-N偶联。在这些理论结果的指导下,我们进一步的实验验证表明,在−0.6 V vs RHE下,合成的Ni-N3B催化剂的法拉第效率为51.92%,尿素产率为32.30 mmol h-1 g-1。我们的工作不仅确定了一种高效的尿素合成催化剂,而不依赖于反复试验的方法,而且还激发了对基于icps的催化剂在电催化中的进一步探索。本文章由计算机程序翻译,如有差异,请以英文原文为准。
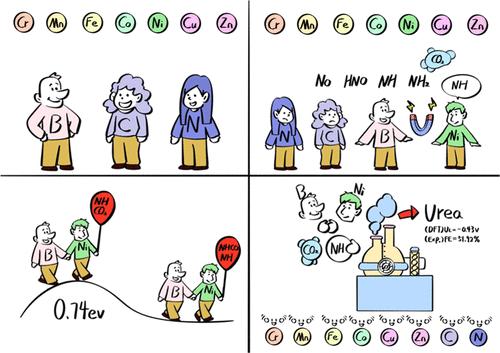
Theory-Guide Design of Integrative Catalytic Pairs for Urea Synthesis from Nitrate and Carbon Dioxide
Electrochemical coreduction of carbon dioxide and nitrate offers a sustainable pathway to synthesize value-added urea from greenhouse gas and nitrogen-containing waste; however, challenges remain in designing efficient catalysts. Based on the concept of “integrative catalytic pairs (ICPs)”, a catalyst for urea synthesis is designed by introducing heteroatoms (B and C) into M–N–C, where a single transition metal is dispersed on N-doped carbon material. Using a two-step theoretical screening strategy, Ni–N3B is identified as a promising catalyst for urea synthesis, with a low limiting potential (−0.43 V) and a small kinetic barrier for C–N coupling (0.74 eV) due to the electronic regulation effects and the synergy of Ni and B function for enhancing NO3– activation and facilitating C–N coupling between gaseous CO2 and *NH intermediate. Under the guidance of these theoretical results, our further experimental validation demonstrates that the synthesized Ni–N3B catalyst achieves a Faradaic efficiency of 51.92% and a urea yield rate of 32.30 mmol h–1 g–1 at −0.6 V vs RHE. Our work not only identifies an efficient urea synthesis catalyst without relying on trial-and-error methods but also inspires further exploration of ICPs-based catalysts in electrocatalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


