代谢可降解π共轭导电聚合物的体内合成,实现无缝神经界面整合和组织修复
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
生物电子学与神经组织的无缝集成对于调节生物信号传递和理解复杂的生理功能至关重要。然而,传统的生物电子材料面临着显著的局限性,包括细胞水平的界面整合差和缺乏可控降解,这阻碍了有效的信号转导和长期的生物相容性。为了解决这些问题,我们开发了一种代谢可降解的π共轭导电聚合物聚吡咯-3-羧酸(PPyCA),并在生理条件下酶促合成。吸电子基团羧酸降低了吡咯环的电子云密度,增强了吡咯环对超氧阴离子的亲和力,从而促进了可控降解。所得到的亲水性PPyCA具有很强的组织亲和力,形成无缝的生物电子界面,并在几个月内完成代谢降解。体内研究表明,酶促合成的PPyCA微泡(MVs)不仅促进神经信号的传递,还能促进损伤后神经的再生。机制研究表明,PPyCA通过MAPK途径上调c-FOS及相关基因的表达,进一步支持其在神经修复中的作用。重要的是,蛋白质组学和代谢组学分析证实没有细胞毒性作用。这项研究为可代谢的电活性聚合物建立了一个新的范例,使无缝的生物电子通信和程序化降解成为可能,为生物医学应用提供了巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
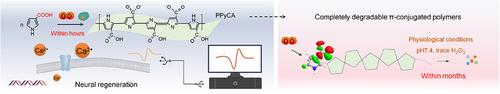
In Vivo Synthesis of Metabolically Degradable π‑Conjugated Conductive Polymers Enabling Seamless Neural Interface Integration and Tissue Repair
The seamless integration of bioelectronics with neural tissues is essential for regulating biological signal transmission and understanding complex physiological functions. However, conventional bioelectronic materials face significant limitations, including poor interfacial integration at the cellular level and a lack of controlled degradation, which hinders effective signal transduction and long-term biocompatibility. To address these challenges, a metabolically degradable π-conjugated conductive polymer, poly(pyrrole-3-carboxylic acid) (PPyCA) is developed and synthesized enzymatically under physiological conditions. The electron withdrawing group, carboxylic acids, reduced the electron cloud density of the pyrrole ring, enhancing the pyrrole ring affinity toward superoxide anion and thereby promoting controlled degradation. The resulting hydrophilic PPyCA has strong tissue affinity, forms seamless bioelectronic interfaces, and undergoes complete metabolic degradation within months. In vivo studies have demonstrated that enzymatically synthesized PPyCA microvesicles (MVs) not only facilitate neural signal transmission but also promote nerve regeneration following injury. Mechanistic investigations revealed that PPyCA upregulates c-FOS and related gene expression through the MAPK pathway, further supporting its role in nerve repair. Importantly, proteomic and metabonomic analyses confirmed the absence of cytotoxic effects. This study establishes a new paradigm for metabolizable electroactive polymers that enable seamless bioelectronic communication and programmed degradation, offering significant potential for biomedical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


