运动学习细化了丘脑对运动皮层的影响
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
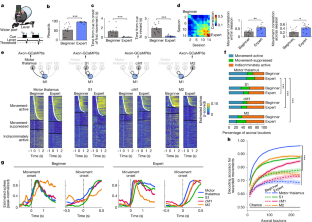
初级运动皮层(M1)是学习和执行灵巧运动技能的中心1,2,3及其浅层(第2层和第3层;因此,L2/3)是学习相关可塑性的关键位点1,4,5,6。目前尚不清楚运动学习如何塑造上游区域激活M1回路来执行学习运动的方式。在这里,利用小鼠M1 L2/3主要输入的纵向轴突成像,我们发现运动丘脑是编码专家(训练两周的动物)学习动作的关键输入源。然后,我们使用光遗传学来识别M1 L2/3神经元的子集,这些神经元在学习前后受到丘脑输入的强烈驱动。我们发现丘脑对M1的影响随着学习而变化,例如运动丘脑优先激活编码专家学习动作的M1神经元。专家对M1的丘脑输入失活会损害习得的动作。我们的研究表明,运动学习重塑了丘脑对M1的影响,使所学运动能够可靠地执行。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Motor learning refines thalamic influence on motor cortex
The primary motor cortex (M1) is central for the learning and execution of dexterous motor skills1–3, and its superficial layer (layers 2 and 3; hereafter, L2/3) is a key locus of learning-related plasticity1,4–6. It remains unknown how motor learning shapes the way in which upstream regions activate M1 circuits to execute learned movements. Here, using longitudinal axonal imaging of the main inputs to M1 L2/3 in mice, we show that the motor thalamus is the key input source that encodes learned movements in experts (animals trained for two weeks). We then use optogenetics to identify the subset of M1 L2/3 neurons that are strongly driven by thalamic inputs before and after learning. We find that the thalamic influence on M1 changes with learning, such that the motor thalamus preferentially activates the M1 neurons that encode learned movements in experts. Inactivation of the thalamic inputs to M1 in experts impairs learned movements. Our study shows that motor learning reshapes the thalamic influence on M1 to enable the reliable execution of learned movements. Imaging and optogenetics in mice provide insight into the interplay between the primary motor cortex and the motor thalamus during learning, showing that thalamic inputs have a key role in the execution of learned movements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


