西妥昔单抗与HFn纳米偶联物的双重靶向策略用于三阴性乳腺癌的免疫治疗
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
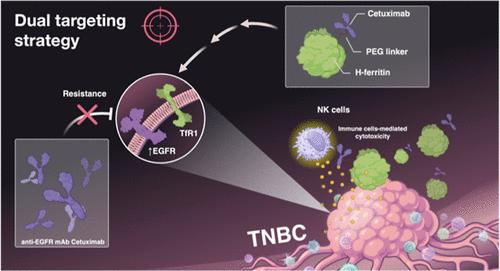
三阴性乳腺癌(TNBC)是一种高度侵袭性和治疗耐药的恶性肿瘤,其特点是缺乏靶向治疗和临床预后差。在这里,我们提出了一种双靶向策略,结合抗egfr单克隆抗体西妥昔单抗(CTX)和h -铁蛋白(HFn),一种靶向转铁蛋白受体1 (TfR1)的纳米颗粒,用于CTX耐药肿瘤的潜在免疫治疗。与单独CTX相比,HFn-CTX纳米偶联物在TNBC球体中表现出良好的生物物理特性和良好的肿瘤积累,并显著增强了抗体依赖性细胞毒性(ADCC)。相反,胶质母细胞瘤球状体没有表现出类似的反应性。这种效应与TNBC细胞中细胞表面EGFR表达的升高和纳米偶联物的质膜滞留有关,促进了强大的免疫激活。生物分布研究表明,HFn-CTX纳米偶联物在TNBC肿瘤中选择性积累。这些发现强调了HFn-CTX纳米偶联物的潜力,可以将CTX重新用于高水平表达EGFR的难治性癌症,如TNBC,利用双受体靶向来增强免疫介导的细胞毒性并克服耐药性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dual-Targeting Strategy to Repurpose Cetuximab with HFn Nanoconjugates for Immunotherapy of Triple-Negative Breast Cancer
Triple-negative breast cancer (TNBC) is a highly aggressive and treatment-resistant malignancy characterized by the lack of targeted therapies and poor clinical outcomes. Here, we present a dual-targeting strategy combining the anti-EGFR monoclonal antibody cetuximab (CTX) with H-ferritin (HFn), a nanoparticle targeting transferrin receptor 1 (TfR1), for potential immunotherapy in CTX-resistant tumors. The HFn–CTX nanoconjugate exhibited favorable biophysical properties and good tumor accumulation and significantly enhanced antibody-dependent cellular cytotoxicity (ADCC) in TNBC spheroids compared to CTX alone. Conversely, glioblastoma spheroids did not exhibit comparable reactivity. This effect correlated with elevated cell-surface EGFR expression and plasma-membrane lingering of the nanoconjugate in TNBC cells, facilitating robust immune activation. Biodistribution studies showed selective accumulation of the HFn–CTX nanoconjugate in TNBC tumors in vivo. These findings highlight the potential of HFn–CTX nanoconjugates to repurpose CTX for refractory cancers that express EGFR at high levels, such as TNBC, leveraging dual-receptor targeting to amplify immune-mediated cytotoxicity and overcome resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


