近红外生物正交激活荧光探针用于肿瘤免疫检查点的体内成像
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
免疫检查点的实时体内成像对肿瘤免疫治疗的指导和预后具有重要意义。尽管可激活光学探针与“永远在线”探针相比具有高特异性和高灵敏度的优点,但由于检查点蛋白通常缺乏酶活性,因此采用基于反应性的设计方法检测检查点蛋白几乎是不可能的。在这里,生物正交反应使荧光开启检测免疫检查点在癌症报道。该方法包括一个生物正交激活的近红外荧光探针(BAP)和一个经环烯(TCO)标记的免疫检查点抗体(αPDL1TCO)。BAP是一种半氰基荧光基团,其羟基被四嗪笼化。在与αPDL1TCO的tco反应后,BAP的四嗪部分被裂解,释放出未被束缚的半菁氨酸,并产生荧光开启反应。BAP不仅可以特异性检测和跟踪治疗期间小鼠结肠癌模型中PDL1表达水平的波动,而且比“始终在线”的荧光基团偶联抗体具有更高的信号-背景比,并且比活检肿瘤组织的流式细胞术分析具有更高的检测灵敏度。预计这种生物正交激活探针的设计策略可以应用于其他无酶反应性的疾病相关蛋白生物标志物的特异性检测。本文章由计算机程序翻译,如有差异,请以英文原文为准。
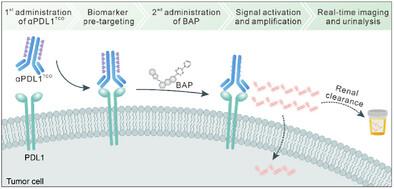
Near-Infrared Bioorthogonally Activatable Fluorescence Probe for In Vivo Imaging of Immune Checkpoint in Cancer
Real-time in vivo imaging of immune checkpoints is important for the guidance and prognosis of cancer immunotherapy. Although activatable optical probes have the advantages of high specificity and sensitivity as compared with “always-on” probes, it is nearly impossible to adopt the reactivity-based design approach for the detection of checkpoint proteins because they generally lack enzymatic activity. Herein, bioorthogonal-reaction-enabled fluorescence turn-on detection of immune checkpoint in cancer is reported. This approach involves a bioorthogonally activatable near-infrared fluorescence probe (BAP) and a transcyclooctene (TCO) tagged immune checkpoint antibody (αPDL1TCO). BAP is a hemicyanine fluorophore whose hydroxyl group is caged by tetrazine. Upon reaction with the TCOs of αPDL1TCO, the tetrazine moiety of BAP is cleaved to release the uncaged hemicyanine with a fluorescence turn-on response. BAP not only allows to specifically detect and track the fluctuation of PDL1 expression level in a murine colon cancer model during therapy but also shows a higher signal-to-background ratio than the “always-on” fluorophore conjugated-antibody, and a higher detection sensitivity than flow cytometric analysis of biopsied tumor tissues. It is expected that the design strategy of this bioorthogonally activatable probe can be applied for specific detection of other disease-related protein biomarkers without enzymatic reactivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


