偶联吡啶配体以铬催化无受体脱氢偶联可持续合成喹啉和吡咯
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了一种简单、原子经济的合成喹啉和吡咯的方法。利用地球上丰富的、市售的CrCl2盐作为催化剂和廉价的、稳定的6,6 ' -二甲基-2,2 ' -二吡啶基作为配体,这种铬催化的无受体脱氢偶联提供了重要的杂环、喹啉和吡咯,合成有用的产率和良好的官能团耐受性,释放水和氢气作为副产物。值得注意的是,所描述的方案也被发现适用于高取代的熔融多环喹啉和吡咯的可持续合成。通过克级喹啉类化合物的合成,验证了该方法的合成价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
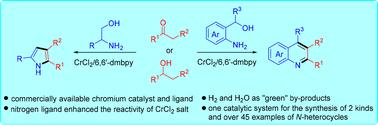
Sustainable synthesis of quinolines and pyrroles enabled by chromium-catalyzed acceptorless dehydrogenative coupling using a bipyridine ligand†
A straightforward and atom-economical method for the synthesis of quinolines and pyrroles has been reported. Using Earth-abundant, commercially available CrCl2 salt as a catalyst and the inexpensive, bench-stable 6,6′-dimethyl-2,2′-dipyridyl as a ligand, this chromium-catalyzed acceptorless dehydrogenative coupling provides important heterocycles, quinolines and pyrroles, in synthetically useful yields with good functional group tolerance, releasing water and hydrogen gas as by-products. Notably, the described protocol was also found to be applicable to the sustainable synthesis of highly substituted fused polycyclic quinolines and pyrroles. Moreover, the synthetic value of this operationally simple protocol was demonstrated by gram-scale synthesis of quinolines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


