结构导向优化2-氨基喹唑啉类造血祖激酶1抑制剂提高口服生物利用度和协同抗肿瘤免疫
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
造血祖激酶1 (HPK1)是一种关键的t细胞免疫抑制因子,具有克服免疫检查点抵抗的转化潜力,但现有的抑制剂无法平衡效力、选择性和药代动力学。我们在我们的2-氨基喹唑啉核心的统一化学框架内开发了一种空间解决策略,整合了(1)HPK1铰链区域亚袋(Leu23/Phe93/Gly95)的高亲和力结合,通过双齿氢键和疏水包装;(2)战略性占领溶剂暴露的变构位点,以立体阻断cyp3a4 / 2c9 / 2d6介导的氧化代谢。优化后的化合物39具有亚纳摩尔结合亲和力(IC50 = 0.70 nM),选择性适中,在人肝微粒体(CLint <;1 mL/min/kg)和良好的口服生物利用度(100%)。在CT26模型中,化合物39通过增加IFN-γ+CD8+肿瘤浸润淋巴细胞(7倍)和增加脾脏IFN-γ产生(3倍)与抗pd -1协同作用(60%的肿瘤生长抑制)。这项工作验证了2-氨基喹唑啉是一种新的HPK1化学型,可解决代谢不稳定性──激酶药物发现的关键障碍。本文章由计算机程序翻译,如有差异,请以英文原文为准。
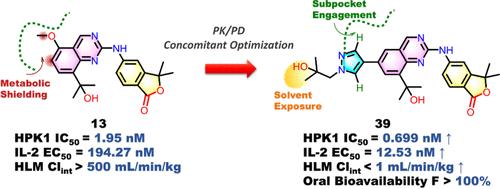
Structure-Guided Optimization of 2-Aminoquinazoline Hematopoietic Progenitor Kinase 1 Inhibitors for Improved Oral Bioavailability and Synergistic Antitumor Immunity
Hematopoietic progenitor kinase 1 (HPK1), a pivotal T-cell immunity suppressor, offers transformative potential to overcome immune checkpoint resistance, yet existing inhibitors fail to balance potency, selectivity, and pharmacokinetics. We developed a spatially resolved strategy within a unified chemical framework of our 2-aminoquinazoline core, integrating (1) high-affinity engagement of the HPK1 hinge-region subpocket (Leu23/Phe93/Gly95) through bidentate hydrogen bonding and hydrophobic packing with (2) strategic occupation of a solvent-exposed allosteric site to sterically block CYP3A4/2C9/2D6-mediated oxidative metabolism. Optimized compound 39 demonstrated subnanomolar binding affinity (IC50 = 0.70 nM) with moderate selectivity, combined with high metabolic stability in human liver microsomes (CLint < 1 mL/min/kg) and favorable oral bioavailability (>100%) in mice. In CT26 models, compound 39 synergized with anti-PD-1 (60% tumor growth inhibition) by expanding IFN-γ+CD8+ tumor-infiltrating lymphocytes (7-fold) and enhancing splenic IFN-γ production (3-fold). This work validates 2-aminoquinazolines as a novel HPK1 chemotype addressing metabolic instability─a key hurdle in kinase drug discovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


