具有三倍放大芬顿样反应的液态金属基纳米复合材料用于肿瘤化学动力学/光热/免疫治疗
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
化学动力治疗(CDT)联合免疫治疗已成为一种很有前景的肿瘤治疗策略。然而,CDT的低效率和免疫抑制肿瘤微环境(ITM)限制了该方法的整体有效性。在本研究中,为了提高CDT和反ITM的效率,开发了一种锰掺杂的介孔二氧化硅包覆的液态金属纳米复合材料,并包覆了小尺寸的金(Au)纳米粒子(NPs)和1-甲基色氨酸(1MT)(标记为LMSMA1)。首先,LMSMA1 NPs可以消耗细胞内过表达的谷胱甘肽(GSH),促进芬顿样离子转移,同时减少GSH介导的活性氧(ROS)清除,从而增强CDT。其次,细胞内葡萄糖被葡萄糖氧化酶(GOx)-模拟Au NPs氧化成过氧化氢(H2O2),提供H2O2促进,进一步放大CDT。此外,LMSMA1 NPs在近红外(NIR)激光照射下提高肿瘤局部温度,实现光热治疗(PTT)增强CDT。由此产生的三重放大芬顿样反应诱导显著的细胞内氧化应激,导致肿瘤细胞死亡。在免疫治疗中,cGAS-STING通路的激活和锰离子(Mn2+)和PTT/CDT引起的免疫原性细胞死亡(ICD)可引起免疫应答,随后激活细胞毒性T细胞。同时,释放的1MT抑制吲哚胺2,3-双加氧酶(IDO),抑制调节性T细胞的功能。这反过来又减轻了免疫抑制微环境,增强了抗肿瘤免疫应答。总之,这一策略建立了一个高度可行的概念,将三扩增CDT与免疫治疗相结合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
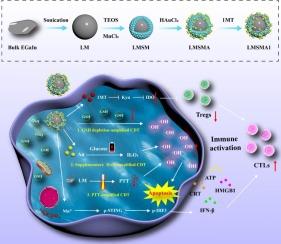
Liquid metal-based nanocomposite with triple-amplified Fenton-like reaction for tumor chemodynamic/photothermal/immunotherapy
Chemodynamic therapy (CDT) combined with immunotherapy has emerged as a promising strategy for tumor treatment. However, the inefficiency of CDT and immunosuppressive tumor microenvironment (ITM) limit the overall effectiveness of this approach. In this study, a manganese-doped mesoporous silica-coated liquid metal nanocomposite, coloaded with small-sized aurum (Au) nanoparticles (NPs) and 1-methyltryptophan (1MT) (denoted as LMSMA1), was developed to improve the efficiency of CDT and reverse ITM. First, the LMSMA1 NPs could consume intracellular overexpressed glutathione (GSH), facilitating Fenton-like ion transfer while reducing GSH-mediated clearance of reactive oxygen species (ROS), thereby enhancing CDT. Second, the intracellular glucose was oxidized to hydrogen peroxide (H2O2) by glucose oxidase (GOx)-mimicking Au NPs, providing an H2O2 boost that further amplified CDT. Additionally, the LMSMA1 NPs increased the local tumor temperature upon near-infrared (NIR) laser irradiation, achieving photothermal therapy (PTT)-enhanced CDT. The resulting triple-amplified Fenton-like reaction induced significant intracellular oxidative stress to cause tumor cell death. For immunotherapy, the activation of cGAS-STING pathway and immunogenic cell death (ICD) resulting from manganese ions (Mn2+) and PTT/CDT could elicit an immune response and subsequently activate cytotoxic T cells. Simultaneously, the released 1MT inhibited indoleamine 2,3-dioxygenase (IDO), suppressing the function of regulatory T cells. This, in turn, alleviated the immunosuppressive microenvironment and reinforced the anti-tumor immune response. In summary, this strategy established a highly feasible concept for integrating triple-amplified CDT with immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


