蒽醌取代基调控iHOFs中离子转运位点以实现碱金属离子的高效转运
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
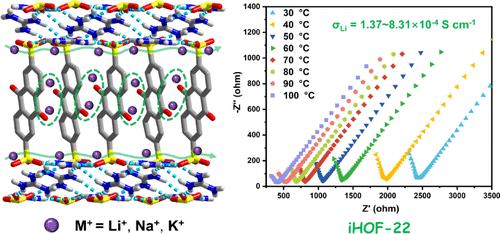
晶体框架材料的可设计性为开发具有高离子电导率的稳定固态电解质提供了希望。在这项研究中,我们提出了三个基于蒽醌的离子氢键有机框架(iHOF-21-23),它们具有二维三明治形氢键网络。通过调节蒽醌取代基,这三种ihof表现出不同的离子转运位点。具体来说,iHOF-22结构中的两个羰基都是离子传输位点,而iHOF-21和iHOF-23各只有一个羰基是离子传输位点,因此iHOF-22表现出更高的离子电导率。iHOF-22在30℃时Li+、Na+和K+的离子电导率分别为1.37 × 10-4、1.14 × 10-4和9.76 × 10-5 S cm-1,比iHOF-21和iHOF-23高16-47%。多维离子输运ihof的设计为各种离子电池的固态电解质的开发提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Anthraquinone Substituents Regulate the Ion-Transport Sites in iHOFs for Efficient Transport of Alkali-Metal Ions
The designability of crystalline framework materials holds promise for the development of stable solid-state electrolytes with high ionic conductivity. In this study, we present three anthraquinone-based ionic hydrogen-bonded organic frameworks (iHOF-21–23), which possess 2D sandwich-shaped hydrogen-bonding networks. By modulating the anthraquinone substituents, the three iHOFs exhibit distinct ion-transport sites. Specifically, both carbonyls in the structure of iHOF-22 are ion-transport sites, whereas only one carbonyl in each of iHOF-21 and iHOF-23 is, and thus iHOF-22 exhibits higher ionic conductivities. The ionic conductivities of Li+, Na+, and K+ of iHOF-22 at 30 °C are 1.37 × 10–4, 1.14 × 10–4, and 9.76 × 10–5 S cm–1, respectively, which are 16–47% higher than those of iHOF-21 and iHOF-23. The design of the iHOFs for multidimensional ion transport provides valuable insights for the development of solid-state electrolytes for various ion batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


