设计双活性中心高效电合成H2O2,打破双电子水氧化中的结垢关系
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
双电子水氧化反应(2e-WOR)是一种非贵重且环境友好的电催化剂,如SnO2,它有望取代传统的能源密集型蒽醌工艺合成贵重的过氧化氢(H2O2),但由于活性位点的中间吸附固有的结垢限制,它的活性和选择性较差。本文报道了一种理论指导的SnO2量子点双活性中心工程,通过引入氧空位(OV)和Zn掺杂剂(ZnD)来打破尺度限制,从而促进了2 - wor。包括operando红外光谱、同位素标记质谱、准原位电子顺磁共振、119Sn Mössbauer光谱和x射线吸收光谱等物理化学表征以及理论计算表明,OV激活水分子将其解离为*OH, ZnD促进随后的*OH偶联,共同促进H2O2的产生。结果表明,在200 mA cm-2条件下,Zn/ SnO2-x的法拉第效率为87.5%,生成速率为52.7 μmol cm-2 min-1,生成H2O2的稳定性为60 h,优于目前报道的大多数e- wor电催化剂。此外,现场生成的H2O2可作为环丙沙星污染物降解和丙烯选择性氧化制丙二醇原料的典型氧化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
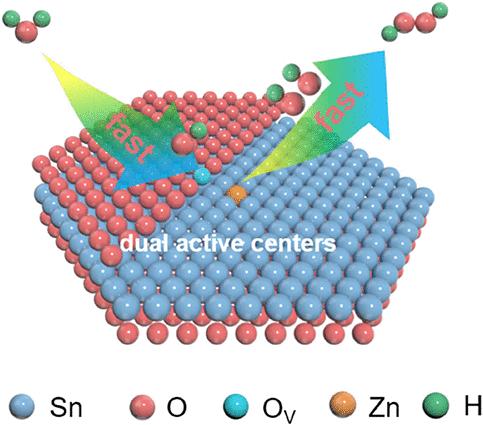
Breaking the Scaling Relationship in Two-Electron Water Oxidation via Designing Dual Active Centers for Efficient H2O2 Electrosynthesis
A two-electron water oxidation reaction (2e-WOR) over nonprecious and environmentally friendly electrocatalysts like SnO2 holds great promise to replace the traditionally energy-intensive anthraquinone process for valuable hydrogen peroxide (H2O2) synthesis, while it is subjected to poor activity and selectivity due to the inherent scaling limitation of intermediate adsorption on active sites. Herein, we report a theory-guided dual active center engineering of SnO2 quantum dots, achieved by introducing oxygen vacancy (OV) and Zn dopant (ZnD), to break the scaling limitation for promoting 2e-WOR. Physicochemical characterizations, including operando infrared spectroscopy, isotope-labeling mass spectrometry, quasi in situ electron paramagnetic resonance, 119Sn Mössbauer spectroscopy, and X-ray absorption spectroscopy, along with theoretical calculations, unveil that OV activates the water molecule to dissociate it to *OH, and ZnD facilitates the subsequent *OH coupling, collectively boosting H2O2 production. Consequently, the resulting Zn/SnO2–x exhibits a high Faradaic efficiency of 87.5% at 200 mA cm–2, a fast production rate of 52.7 μmol cm–2 min–1, and robust stability of 60 h for H2O2 generation, superior to most reported 2e-WOR electrocatalysts. In addition, the on-site generated H2O2 can be used as a typical oxidant for ciprofloxacin pollutant degradation and selective propylene oxidation to propylene glycol feedstock.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


