以氢氧阴离子为催化剂的离子多孔有机聚合物凝胶,用于胺和酰胺与二氧化碳†的n -甲酰化反应
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
温室气体CO2的减量化和功能化符合绿色可持续发展的特点。在温和条件下,通过三胺胍与1,1′-(对苯二亚甲基)双(4-甲酰基吡啶)阳离子单元的腙缩合反应制备了离子多孔有机聚合物凝胶。通过离子交换制备了一种具有氢氧阴离子、多离子活化中心和多孔凝胶结构的无金属三氨基胍-吡啶复合多孔有机聚合物凝胶(PT-OH)。PT-OH具有优异的官能团耐受性和高活性,可在室温和1atm CO2压力下以苯硅烷为还原剂实现伯胺、仲胺和酰胺的n -甲酰化反应。此外,PT-OH具有化学稳定性,易于回收,可重复使用5次而不损失活性。该材料代表了一种高效、经济、环保的非均相金属催化体系,符合二氧化碳绿色利用的原则。本文章由计算机程序翻译,如有差异,请以英文原文为准。
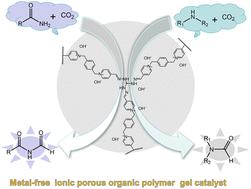
An ionic porous organic polymer gel with hydroxide anions as an efficient catalyst for N-formylation of amines and amides with carbon dioxide†
The reduction and functionalization of greenhouse gas CO2 remain in line with the characteristics of green and sustainable development. In this work, an ionic porous organic polymer gel was prepared through a hydrazone condensation reaction between triaminoguanidine and 1,1′-(p-phenylenedimethylene)bis(4-formylpyridinium) cationic units under mild conditions. A metal ion-free triaminoguanidine- and pyridinium-incorporated porous organic polymer gel (PT-OH) with triple synergistic effects of hydroxide anions, multi-ion activation centers and a porous gel structure was obtained after ion exchange. PT-OH provides excellent functional group tolerance and high activity to achieve the N-formylation reaction of primary amines, secondary amines and amides using phenylsilane as the reducing agent under ambient conditions (room temperature and 1 atm CO2 pressure). Moreover, PT-OH has chemical stability, is easy to recycle and can be reused 5 times without loss of activity. The material represents an efficient, economical and environmentally friendly heterogeneous metal-free catalytic system, which conforms to the principle of green utilization of CO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


