氧空位介导的过氧乙酸活化选择性生成1O2用于水净化
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
作为一种预氧化单元,开发出氧化效率高、环境稳健性强、生态安全的非自由基途径主导的高级氧化工艺(AOPs)是实际水污染治理的必要条件。本文以Co3O4为例,提出了一个氧空位(OVs)介导的过氧乙酸(PAA)活化过程,从而主要产生单线态氧(1O2)来降解污染物。对富含ovs的Co3O4 (Co3O4- ovs)活化PAA的现场监测表明,表面含氧中间体(如*OH和*O)是1O2的前体。理论计算表明,末端氧原子(ATO)在PAA中的选择性吸附是生成1O2的活性描述符。OVs可诱导电子重分布,触发ato主导的PAA吸附形成Co3O4-OVs-PAA*络合物,O-O键断裂生成*OH。同时,OVs调节Co - d波段中心,降低形成1O2的能垒。该系统具有超快的催化性能(kobs = 1.17 min-1),可降解磺胺甲恶唑,性能比原始Co3O4高11.64倍。对非自由基途径的高选择性使Co3O4-OVs/PAA体系在复杂环境背景和连续流微反应器中具有显著的稳定性。这项工作不仅为通过缺陷工程调节非自由基途径提供了广阔的视角,而且还推动了paas基AOPs用于水净化的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。

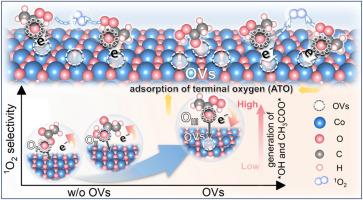
Oxygen vacancies-mediated the peracetic acid activation to selectively generate 1O2 for water decontamination
As a pre-oxidation unit, developing non-radical pathway-dominant advanced oxidation processes (AOPs) with remarkably-efficient oxidation, superior environmental robustness, and ecological safety is essential in actual water pollution control. Herein, using Co3O4 as an example, we present an oxygen vacancies (OVs)-mediated peracetic acid (PAA) activation process, thereby predominantly generating singlet oxygen (1O2) for degrading contaminants. In-situ monitoring of PAA activation by OVs-rich Co3O4 (Co3O4-OVs) reveals that surface oxygen-containing intermediates (e.g., *OH and *O) are the precursors of 1O2. Theoretical calculations show that the selective adsorption of terminal oxygen atoms (ATO) in PAA serves as an activity descriptor for 1O2 generation. OVs can induce electron redistribution, triggering the ATO-dominated PAA adsorption to form the Co3O4-OVs-PAA* complex, followed by O-O bond breakage to yield *OH. Concurrently, OVs modulate the Co d-band center, lowering the energy barrier for 1O2 formation. The system enables ultra-fast catalytic performance (kobs = 1.17 min–1) for degrading sulfamethoxazole, outperforming pristine Co3O4 by 11.64-fold. The high-selectivity towards non-radical pathway endows the Co3O4-OVs/PAA system with remarkable stability in complex environment backgrounds and continuous-flow microreactor. This work not only provides a broad perspective on the modulation of non-radical pathways via defect engineering, but also advances the development of PAA-based AOPs for water decontamination.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


