平面氯化工程增强Fe-N4位极性以促进硝酸盐电还原
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在传统的金属- n4催化位点引入极性,加强极性NO2*中间体的吸附,有利于硝酸盐深度还原成氨。其中,平面氯化工程策略成功地在Fe-N4催化位点附近引入了极性更高的C-Cl键,有效地提高了硝酸盐还原反应(NO3RR)的催化活性。在−0.83 V / RHE条件下,Fe-N4 /CNCl催化剂催化NH3的最大产率为1.82 mg h-1 cm-2, TOF值为245 h-1,分别是传统无极性Fe-N4 /CN催化剂的3.4倍和2.7倍。Fe-N4 /CNCl催化剂在连续15次循环中也表现出令人满意的稳定性。密度泛函理论(DFT)计算表明,高极性Fe-N4位点的平面氯化工程增强了极性NO2*中间体的吸附,促进了硝酸盐深度还原成氨,降低了决定速率步骤(RDS)的能垒,从而提高了催化活性。这项工作展示了平面氯化工程策略的巨大优势,通过在传统的金属- n4催化位点引入极性来提高电催化活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
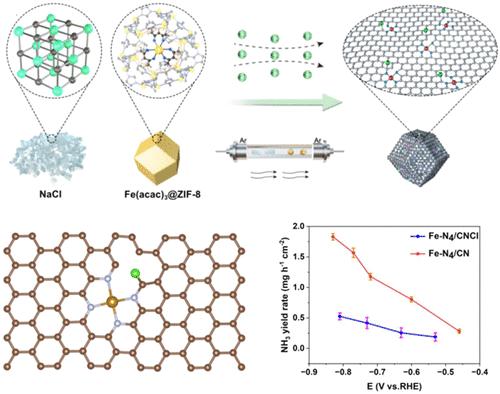
Planar Chlorination Engineering Enhances the Polarity of the Fe–N4 Site for Boosting Nitrate Electroreduction
Introducing polarity in traditional metal-N4 catalytic sites and strengthening the adsorption of polar NO2* intermediate are advantageous for the deep reduction of nitrate into ammonia. Herein, the planar chlorination engineering strategy successfully introduced the C–Cl bonds adjacent to the Fe–N4 catalytic site with higher polarity, effectively boosting the catalytic activity for the nitrate reduction reaction (NO3RR). The maximal NH3 yield rate and the corresponding turnover frequency (TOF) value catalyzed by the Fe–N4/CNCl catalyst were 1.82 mg h–1 cm–2 and 245 h–1 at −0.83 V vs RHE, which were 3.4 times and 2.7 times those of the traditional Fe–N4/CN catalyst without polarity, respectively. The Fe–N4/CNCl catalyst also exhibited satisfactory stability during consecutive 15 cycles. The density functional theory (DFT) calculation revealed that the planar chlorination engineering of the Fe–N4 site with higher polarity strengthened the adsorption of polar NO2* intermediate, facilitated the deep reduction of nitrate into ammonia, lowered the energy barrier of rate-determining step (RDS) and thus improved the catalytic activity. This work exhibited the enormous advantage of a planar chlorination engineering strategy for enhancing the activity of electrocatalysis by introducing polarity in the traditional metal-N4 catalytic site.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


