Picoplatin的Diiodido类似物的细胞毒性和与DNA、溶菌酶、核糖核酸酶A和人血清白蛋白的结合
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
在这里,我们研究了picoplatin的碘类似物,顺胺-二碘(2-甲基吡啶)铂(II)配合物(I-picoplatin)的细胞毒性和DNA和蛋白质结合。i - pico铂(IC50 = 3.7 ~ 12.4 μM)优于pico铂(IC50 = 11.8 ~ 22.6 μM),对A2780卵巢癌细胞的顺铂耐药能力优于pico铂。与picoplatin和顺铂相比,I-picoplatin在HeLa宫颈癌细胞中诱导不同的细胞周期变化(减少s期分数和增加G2/M期阻滞)。用溴化乙啶置换法和圆二色法研究了金属化合物与DNA模型系统的结合。用x射线衍射和质谱法研究了其与溶菌酶(HEWL)和胰腺RNase A的反应性。i - pico铂与DNA双螺旋结合,当与两种蛋白质结合时,能够保留2-甲基吡啶配体和至少一种碘配体。各种含pt的基团,包括一个基于i - pico铂的异构化结构,协调His和Met残基。i - pico铂/人血清白蛋白(HSA)加合物的低分辨率结构也得到了解决。His146、Met289和Met329的侧链是i - pico铂在HSA上的主要结合位点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
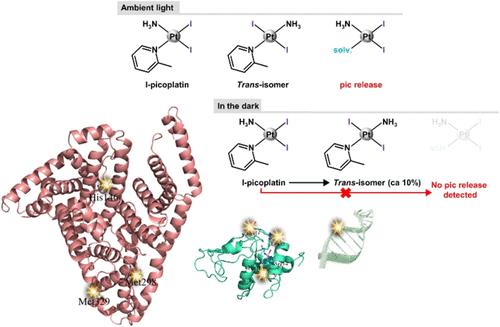
Cytotoxicity and Binding to DNA, Lysozyme, Ribonuclease A, and Human Serum Albumin of the Diiodido Analog of Picoplatin
Here we investigated cytotoxicity and DNA and protein binding of an iodido analog of picoplatin, the cis-ammine-diiodido(2-methylpyridine)platinum(II) complex (I-picoplatin). I-picoplatin (IC50 = 3.7–12.4 μM) outperforms picoplatin (IC50 = 11.8–22.6 μM) in the human cancer cell lines used and shows a greater ability to overcome the cisplatin resistance of A2780 ovarian cancer cells than does picoplatin. I-picoplatin also induces different cell cycle changes (reduced S-phase fraction and an increase in the G2/M phase arrest) in HeLa cervical carcinoma cells compared to both picoplatin and cisplatin. Binding of the metal compound to DNA model systems was investigated by ethidium bromide displacement assay and circular dichroism. Its reactivity with lysozyme (HEWL) and pancreatic RNase A was studied by X-ray diffraction and mass spectrometry experiments. I-picoplatin binds the DNA double helix and is able to retain the 2-methylpyridine ligand and at least one of the two iodido ligands when bound to the two proteins. Various Pt-containing moieties, including one based on the isomerized structure of I-picoplatin, coordinate the His and Met residues. A low-resolution structure of the I-picoplatin/human serum albumin (HSA) adduct has also been solved. The side chains of His146, Met289, and Met329 are the primary binding sites of the I-picoplatin moieties on HSA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


