丙炔醇的电化学氧化:在温和条件下快速获得前所未有的二氧正酯
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
研究了一种新型的、环境友好的、无金属的方法,通过电化学氧化丙炔醇来合成独特的二氧基邻苯二甲酸酯衍生物。这种直接的方法有效地将易获得的具有末端炔的s-丙炔醇转化为二氧邻苯二甲酸酯,这是一种尚未开发的化合物。该方法操作简便,原料便宜,衬底范围广,具有一定的吸引力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
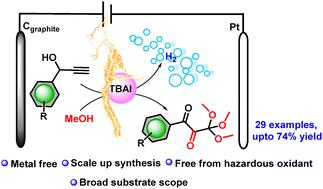
Electrochemical oxidation of propargyl alcohol: rapid access to an unprecedented dioxo-orthoester under mild conditions†‡
A novel, environmentally benign, metal-free method has been developed for synthesizing unique dioxo-orthoester derivatives through electrochemical oxidation of propargyl alcohols. This straightforward approach efficiently converts readily accessible s-propargyl alcohols with terminal alkynes into dioxo-orthoesters, which constitute an unexplored class of compounds. The operational ease, inexpensive starting materials and broad substrate scope make this method appealing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


