调节TM-O键共价提高Na4Fe1.5Mn1.5(PO4)2P2O7的阳离子活性和可逆性
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
对成本效益的追求激发了人们对Na4Fe1.5Mn1.5(PO4)2P2O7 (NMFPP)阴极的极大兴趣。然而,它的阳离子氧化还原活性和可逆性很难达到预期,并且导电性差,结构降解快。这些问题可归因于TM-O键在聚阴离子晶体场中的高电离度,加剧了电子局域化,降低了TMO6八面体在Jahn-Teller效应下的稳定性。本文提出了一种提高TM-O键共价的策略。具体来说,引入具有强亲电性的Ti4+改变TM-O键的局部电子结构,包括能带结构和键强度。最终,Ti修饰的Na4Mn1.3Fe1.5Ti0.1(PO4)2P2O7 (NMFTPP)的固有电导率和晶格稳定性得到了很好的优化,提高了阳离子氧化还原的活性和可逆性。本工作揭示了TM-O键共价与聚阴离子材料本征电导率/结构稳定性之间的潜在机制,为钠离子电池的高性能发展开辟了一条可行的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
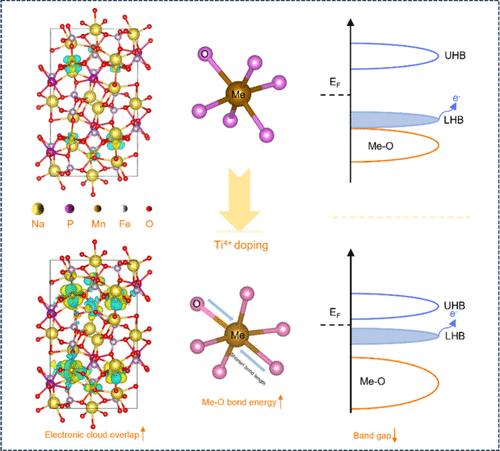
Tuning TM-O Bond Covalency to Boost Cationic Activity and Reversibility of Na4Fe1.5Mn1.5(PO4)2P2O7
The pursuit of cost-effectiveness stimulates great interest in the Na4Fe1.5Mn1.5(PO4)2P2O7 (NMFPP) cathode. However, its cationic redox activity and reversibility are hardly up to expectation, accompanied by poor conductivity and rapid structural degradation. These issues can be attributed to the high ionization degree of TM-O bonds in the polyanion crystal field, which intensifies electronic localization and degrades the stability of TMO6 octahedra under the Jahn–Teller effect. Herein, a strategy is proposed to enhance the covalency of TM-O bonds. Specifically, Ti4+ with strong electrophilicity is introduced to alter the local electronic structure of TM-O bonds, including band structure and bonding strength. Ultimately, both intrinsic conductivity and lattice stability of Ti modified Na4Mn1.3Fe1.5Ti0.1(PO4)2P2O7 (NMFTPP) are well optimized, upgrading the activity and reversibility of cationic redox. This work reveals the potential mechanism between TM-O bond covalency and the intrinsic conductivity/structural stability of polyanion materials, opening up a feasible path for the high-performance development of sodium ion batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


