基于c端活性残基自适应和修饰的水蛭素衍生的抗凝血性能增强肽的构建
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
水蛭素通过抑制凝血酶与纤维蛋白原的结合和催化活性来发挥抗凝血作用。然而,它的刚性n端区域不可逆地占据凝血酶催化中心,引起对潜在出血的担忧。目的基于水蛭素变体WPHV_C的竞争结合机制,制备一种新的先导化合物WPHVC_V1,并对其体外、体内活性和安全性进行验证。方法采用饱和诱变、分子动力学模拟和突变蛋白活性分析等方法研究WPHV_C与凝血酶的竞争抗凝机制。接下来,利用重组表达的酪氨酸蛋白硫转移酶对水蛭素c端酪氨酸的硫化位点进行修饰和确认。最后,采用多位点芳香族氨基酸突变策略,设计合成了先导抗凝剂WPHVC_V1。结果WPHV_C中的酸性氨基酸团簇与带正电荷的凝血酶外源I形成强静电相互作用,阻断纤维蛋白原结合。芳香族氨基酸的引入通过π-π叠加和π-阳离子相互作用进一步稳定了配合物。例如,13E突变为A使解离自由能(ΔG)从19.27降低到10.93 kcal·mol−1,凝血酶时间(TT)从42.00 s缩短到30.94 s,而26 K突变为W使ΔG增加到24.70 kcal·mol−1,使TT延长到51.92 s。此外,20Y的芳香效应,与磺化结合,协同增强结合。在此基础上,新设计的WPHVC_V1的TT/APTT/PT (WPHV_C)从41.72/14.38/15.86 s (WPHV_C)提高到62.08/23.38/22.22 s(0.1 mg/ml), ΔG为37.24 kcal·mol−1。在体内研究中,WPHVC_V1通过将CK中的血栓长度从3.562 cm减少到1.853 cm(肝素钠为1.729 cm, WPHV_C为2.530 cm)实现了小鼠尾部血栓的抑制,完全抑制了颈动脉模型中的血栓形成,与肝素钠相比,尾部出血时间缩短了35.2 s。安全性评价显示,WPHVC_V1无溶血作用,对血压无明显影响,对主要脏器无病理改变。结论本研究结果为开发安全有效的抗凝药物提供了初步的基础和序列参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
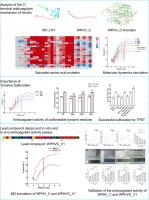
Construction of safer hirudin-derived peptides with enhanced anticoagulant properties based on C-terminal active residue adaptation and modification
Introduction
Hirudin exerts anticoagulant effects by inhibiting the binding and catalytic activity of thrombin to fibrinogen. However, its rigid N-terminal region irreversibly occupies the thrombin catalytic center, raising concerns about potential bleeding.Objectives
In this study, a novel lead compound, WPHVC_V1, which is based on the competitive binding mechanism of the hirudin variant WPHV_C, was developed and validated for in vitro and in vivo activity and safety.Methods
Saturation mutagenesis, molecular dynamics simulations and mutant protein activity assays were used to elucidate the competitive anticoagulant mechanism between WPHV_C and thrombin. Next, a recombinantly expressed tyrosylprotein sulfotransferase was used to modify and confirm the sulfation site on the C-terminal tyrosine of hirudin. Finally, a multisite aromatic amino acid mutation strategy was implemented to design and synthesize the lead anticoagulant, WPHVC_V1.Results
The acidic amino acid cluster in WPHV_C formed strong electrostatic interactions with the positively charged thrombin exosite I, blocking fibrinogen binding. The introduction of aromatic amino acids further stabilized the complex through π-π stacking and π-cation interactions. For example, mutation of 13E to A decreased the free energy of dissociation (ΔG) from 19.27 to 10.93 kcal·mol−1 and shortened the thrombin time (TT) from 42.00 s to 30.94 s, whereas mutation of 26 K to W increased the ΔG to 24.70 kcal·mol−1 and prolonged TT to 51.92 s. In addition, the aromatic effect of 20Y, combined with sulfation, synergistically enhanced binding. Based on these findings, the newly designed WPHVC_V1 showed a ΔG of 37.24 kcal·mol−1 and, at 0.1 mg/ml, increased TT/APTT/PT from 41.72/14.38/15.86 s (WPHV_C) to 62.08/23.38/22.22 s. In in vivo studies, WPHVC_V1 achieved tail thrombus inhibition in the mouse tail by reducing the length of the thrombus from 3.562 cm in CK to 1.853 cm (1.729 cm for sodium heparin and 2.530 cm for WPHV_C), completely inhibited thrombus formation in a carotid artery model and reduced tail bleeding time by 35.2 s compared with heparin sodium. Safety evaluations revealed that WPHVC_V1 did not cause hemolysis, had no significant effect on blood pressure or cause pathological changes in major organs.Conclusion
These findings provide an initial foundation and sequence reference for the development of safe and effective anticoagulant drugs with potential for clinical translation.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


