衬底-光催化剂反应性匹配使芳基卤化物范围在光驱动,还原性交叉亲电偶联使用13C核磁共振作为预测因子
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
芳基卤化物和烷基卤化物之间的亲电偶联是一种重要的合成工具,可以从丰富的构建块中生成Csp2-Csp3键。这些联轴器传统上依赖于热驱动的还原性联轴器,使用金属Zn/Mn来产生活性的低价镍。最近的工作将这种反应性扩展到利用一系列光催化剂和末端还原剂的可见光驱动方法。然而,早期的光催化方法需要使用贵金属光催化剂和昂贵的硅烷的化学计量量。本文描述的工作扩展了光氧化还原催化的交叉亲电偶联,重点是使用有机光催化剂和一种廉价、容易获得的牺牲电子源(三乙醇胺)。为了克服由光催化剂和底物之间的氧化还原不相容引起的底物范围的限制,我们引入了两组条件,以最大限度地减少不必要的底物特异性副反应性。这些合成方案使具有挑战性的芳基溴底物,如无保护的溴吲哚和2-溴嘧啶的交叉亲电偶联成为可能。我们发现芳基卤化物试剂发生副反应的倾向与电子参数相关:芳基溴的C-Br 13C化学位移是这种反应性的可靠预测因子,并使反应条件易于选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
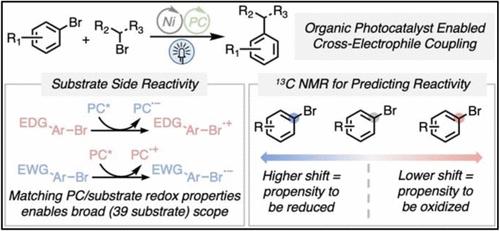
Substrate-Photocatalyst Reactivity Matching Enables Broad Aryl Halide Scope in Light-Driven, Reductive Cross-Electrophile Coupling Using 13C NMR as a Predictor
The cross-electrophile coupling between aryl and alkyl halides is an important synthetic tool in the creation of Csp2–Csp3 bonds from abundant building blocks. These couplings have traditionally relied on thermally driven, reductive couplings using metallic Zn/Mn to generate reactive low-valent nickel species. Recent work has expanded this reactivity to visible light-driven methodologies utilizing a range of photocatalysts and terminal reductants. However, early photocatalyzed approaches required the use of precious metal photocatalysts and stoichiometric amounts of a costly silane. The work described herein expands photoredox catalyzed cross-electrophile coupling with a focus on the use of organic photocatalysts and an inexpensive, readily available sacrificial electron source (triethanolamine). To overcome limitations in substrate scope arising from redox incompatibilities between photocatalyst and substrate, we introduce two sets of conditions that minimize unwanted substrate-specific side reactivity. These synthetic protocols enable cross-electrophile couplings of challenging aryl bromide substrates, such as unprotected bromoindoles and 2-bromopyrimidines. We found that the propensity of aryl halide reagents to undergo side reactions is correlated with electronic parameters: the C–Br 13C chemical shift of aryl bromides is a robust predictor for this reactivity and enables facile reaction condition selection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


