蒙脱土吸附镭的机理:DFT和实验研究
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
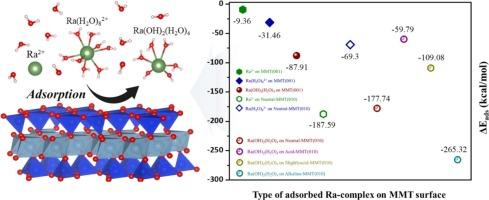
从乏燃料中释放的镭(Ra)由于其长半衰期(1600 年)和放射性危害而构成挑战,但其稀有性限制了实验研究。本文采用理论计算和实验相结合的方法,探讨了工程屏障中重要的膨润土组分蒙脱土对Ra的吸附机理。理论结果表明,在不同水和羟基配位数的水溶液中,有3种稳定的ra配合物。研究结果表明,Ra(OH)2(H2O)4在(0 0 1)表面形成内球配合物,表现出比Ra2+和[Ra(H2O)8]2+更大的稳定性。在(0 1 0)表面,Ra2+和Ra(OH)2(H2O)4均形成球内配合物,与[Ra(H2O)8]2+相比,Ra2+通过较弱的氢键吸附,具有较强的吸附能力。此外,在高ph下,(0 1 0)表面吸附的Ra(OH)2(H2O)4在脱质子表面表现出最稳定的形态。电荷转移和态密度分析也有效地证明了Ra在蒙脱土中的吸附稳定性。此外,Ra的间歇吸附实验证实了Ra的吸附遵循线性等温线,并且与pH相关的实验表明,在高pH条件下Ra的吸收率最高,与理论结果一致。这些观察结果表明,工程屏障可能会减轻受混凝土和岩石引起的高pH值影响的深层处置库中的Ra运输。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Mechanistic insights into radium adsorption on montmorillonite: DFT and experimental studies
Radium (Ra) released from spent nuclear fuel poses a challenge due to its long half-life (1600 years) and radiological hazard, but its rarity limits experimental studies. This study explores the Ra adsorption mechanism on montmorillonite, a key bentonite component in engineered barriers, using theoretical calculations and experiments. Theoretical results identified three stable Ra-complexes in aqueous solutions with different water and hydroxyl coordination numbers. Our findings demonstrate that Ra(OH)2(H2O)4 forms inner-sphere complexes on (0 0 1) surfaces, exhibiting greater stability than Ra2+ and [Ra(H2O)8]2+ species. On the (0 1 0) surface, both Ra2+ and Ra(OH)2(H2O)4 form inner-sphere complexes with stronger adsorption compared to [Ra(H2O)8]2+, which adsorbs through weaker hydrogen bonding. Additionally, Ra(OH)2(H2O)4 adsorbed on the (0 1 0) surface showed the most stable form on the deprotonated surface at high pH. The stability of Ra adsorption in montmorillonite was also effectively demonstrated by charge transfer and density-of-states analysis. Furthermore, Ra batch adsorption experiments confirmed that Ra adsorption followed a linear isotherm, and pH-dependent experiments showed that Ra uptake was highest at high pH conditions, in agreement with theoretical results. These observations suggest that engineered barriers may mitigate Ra transport in deep disposal repositories subject to high pH induced by concrete and rock.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


