通过酸碱对快速质子转移途径的质子导电有机-无机纳米复合材料
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
全球环境可持续发展的驱动力正在加速向可再生能源技术的过渡,聚合物电解质膜燃料电池(pemfc)由于其卓越的能源效率和紧凑的设计而成为关键的竞争者,尽管传统的基于Nafion®的pemfc面临着严峻的挑战,例如在高温和低湿度环境下成本升高和性能下降,这限制了其更广泛的应用。为了克服这些挑战并满足严格的环境标准,开发无氟电解质膜对于确保在各种操作条件下稳定的导电性至关重要。本研究以(3-甘氧基丙基)三甲氧基硅烷(GPTS)和(3-巯基丙基)三甲氧基硅烷(MPTS) (G-M)为原料,通过溶胶-凝胶法合成了一种无机-有机杂化基质,并分别用(氨丙基)三甲氧基硅烷(APTES)、聚多巴胺(PDA)和壳聚糖(CS)三种不同的碱性添加剂进行了功能化。这些添加剂通过复合聚合物基体中酸性和碱性组分之间的静电相互作用加入,以增强质子的导电性。G-M/APTES、G-M/PDA和G-M/CS复合膜通过基质的-磺酸(-SO3H)基和添加剂的氨基(-NH2)基之间的酸碱配对作用,增强了质子的传输。含20 wt% CS的G-M/CS膜,其通过面质子电导率(σth)最高,为1.59 mS/cm,比原始G-M基质提高了4.68倍。相反,在80°C和100%相对湿度(RH)条件下,用40 wt% PDA功能化的G-M/PDA膜的面内质子电导率(σin)为37.10 mS/cm,相对于原始G-M膜的σin提高了1000多倍。这些结果强调了酸碱相互作用在推进聚合物电解质膜电化学应用方面的有效性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
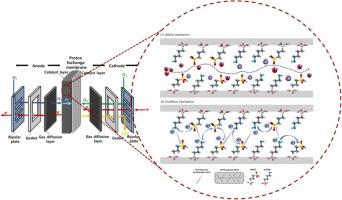
Proton-conducting organic-inorganic nanocomposites with fast proton-transfer pathways through acid-base pairs
The drive for global environmental sustainability is accelerating the transition to renewable energy technologies, with polymer electrolyte membrane fuel cells (PEMFCs) emerging as a key contender due to their superior energy efficiency and compact design, although traditional Nafion®-based PEMFCs face critical challenges, such as elevated costs and diminished performance under high-temperature and low-humidity environments, which limit their broader implementation. To overcome these challenges and meet stringent environmental standards, the development of non-fluorinated electrolyte membranes is essential to ensure stable conductivity across diverse operating conditions. In this study, a hybrid inorganic-organic matrix was synthesized via a sol-gel process by combining (3-glycidoxypropyl)trimethoxysilane (GPTS) and (3-mercaptopropyl)trimethoxysilane (MPTS) (referred as G-M) and subsequently functionalized with three distinct basic additives-(aminopropyl)triethoxysilane (APTES), polydopamine (PDA), and chitosan (CS). These additives were incorporated through electrostatic interactions between acidic and basic components within the composite polymer matrix to enhance proton conductivity. The resulting G-M/APTES, G-M/PDA, and G-M/CS composite membranes exhibited enhanced proton transport through acid-base pairing interactions between the matrix’s –sulfonic acid (-SO3H) groups and additives’ amino (–NH2) groups. The G-M/CS membrane, incorporating 20 wt% CS, exhibited the highest through-plane proton conductivity (σth) of 1.59 mS/cm, corresponding to a 4.68-fold improvement over the pristine G-M matrix. Conversely, the G-M/PDA membrane, functionalized with 40 wt% PDA, achieved a remarkable in-plane proton conductivity (σin) of 37.10 mS/cm under conditions of 80 °C and 100 % relative humidity (RH), reflecting an over thousand-fold enhancement relative to the σin of pristine G-M membranes. These results underscore the efficacy of acid-base interactions in advancing polymer electrolyte membranes for electrochemical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


