高能水质子电池的同位素界面设计
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
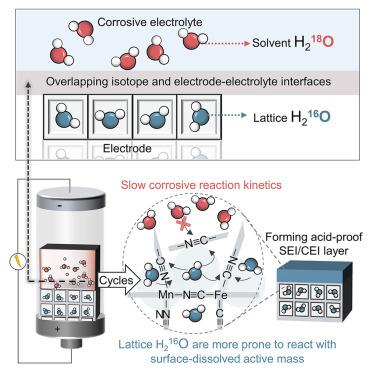
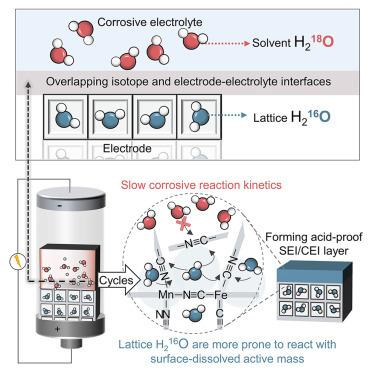
电极-电解质界面的稳定性对于电池化学物质的长期运行至关重要,特别是在腐蚀性酸性/碱性水溶液电解质的恶劣条件下。在这里,我们报告了H218O-H216O同位素界面的设计,以促进保护性电极-电解质界面的形成,并通过基于水质子电池(APB)的模型研究证明了这一点,该电池使用强酸性水溶液电解质。这种设计使锰铁普鲁士蓝类似物(MnFe-PBA),通常被认为不耐酸,在腐蚀性H3PO4电解质中稳定循环超过10,000次。这种耐酸性归因于同位素界面控制的由氢键框架组成的界面相的原位形成。使用高能MnFe-PBA阴极,整个电池达到了高电压平台(⁓1.2 V)和能量密度(77.6 Wh kg−1),两者都超过了之前报道的所有apb。我们的发现为提高在极端腐蚀条件下的水性电池的性能提供了一种新的方法,并鼓励同位素科学与电池技术的整合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Isotope interface design for high-energy aqueous proton batteries
The stability of the electrode-electrolyte interface is crucial for the long-term operation of battery chemistries, particularly under harsh conditions with corrosive acidic/alkaline aqueous electrolytes. Here, we report the design of a H218O-H216O isotope interface to promote the formation of a protective electrode-electrolyte interphase, as demonstrated by a model investigation based on an aqueous proton battery (APB) utilizing strongly acidic aqueous electrolytes. This design enables the manganese-iron Prussian blue analog (MnFe-PBA), typically considered acid intolerant, to cycle stably over 10,000 cycles in corrosive H3PO4 electrolytes. This acid resistance is attributed to the isotope interface-governed in situ formation of interphases comprising hydrogen-bonded frameworks. With the high-energy MnFe-PBA cathode, the full battery attains a high voltage plateau (⁓1.2 V) and energy density (77.6 Wh kg−1), both surpassing all previously reported APBs. Our findings provide a novel approach for enhancing the performance of aqueous batteries subject to extremely corrosive conditions and encourage the integration of isotopic science with battery technology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


