π-键和σ-键的光硫氰胺化:试剂开发及合成应用
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
1,2-硫氰胺构成了一类重要的结构基序,存在于许多生物活性分子和更多生物活性分子的前体中。尽管它们具有合成意义,但对这种非功能化的权宜之计是罕见的。本发明公开了一种硫氰胺化试剂的开发,该试剂利用光电介导的能量转移现象用于烯烃的易硫氰胺化。该策略也适用于σ-键,为获得注入胺和-SCN的小分子提供了一种通用策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
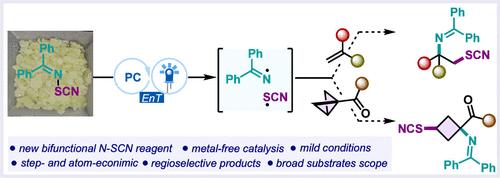
Photo-Thiocyanoamination of π- and σ-Bonds: Reagent Development and Synthetic Applications
1,2-Thiocyanoamines make up a class of important structural motifs that are found in a number of bioactive molecules and precursors to many more. Despite their synthetic significance, expedient access to this difunctionalization is rare. Herein, the development of a thiocyanoimination reagent is disclosed, taking advantage of photomediated energy transfer phenomena for the facile thiocyanoimination of alkenes. The strategy was found to be viable for σ-bonds as well, providing a generalized strategy for accessing small molecules infused with amine and -SCN.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


