微流控集成扫描电化学显微镜对上铁细胞间通讯的原位研究
IF 9.1
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
上睑下垂已被认为是治疗多种癌症的潜在方法。然而,在复杂的肿瘤微环境中,癌细胞与肿瘤相关巨噬细胞(tumor associated macrophages, tam)之间的交流在肿瘤的发生和发展中起着至关重要的作用。在这项工作中,扫描电化学显微镜(SECM)与微流控装置相结合,实现了芯片上细胞共培养和原位研究三阴性乳腺癌细胞(tnbc)与TAMs在铁下垂中的通讯。在共培养系统中,tnbc和tam分别作为应答细胞和信号细胞。通过原位监测erastin诱导的铁死亡关键参数(ROS、谷胱甘肽(GSH)、细胞膜通透性)的变化,发现TAMs部分恢复了TNBC中GSH外排减少、ROS释放增加、细胞膜屏障受损的情况,提示TAMs可以抑制TNBC铁死亡。机制上,tnbc可促进M2巨噬细胞极化,M2- tam通过stat3相关信号通路抑制tnbc铁凋亡。抑制STAT3后,通过SECM原位监测tnbc的ROS释放和膜通透性增加,GSH外排减少,证明了铁下垂的细胞间通讯机制。因此,这项工作提供了一种靶向tam治疗基于铁细胞凋亡的TNBC的潜在策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
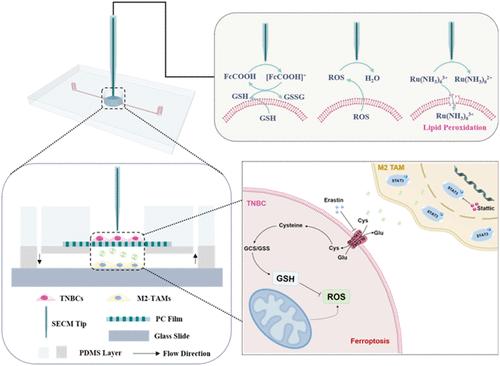
In Situ Investigation of Intercellular Communication in Ferroptosis Integrated Scanning Electrochemical Microscopy with Microfluidic Devices
Ferroptosis has been recognized as a potential treatment for various cancers. Still, in the complex tumor microenvironment, the communication between cancer cells and tumor-associated macrophages (TAMs) plays a crucial role in tumorigenesis and progression. In this work, scanning electrochemical microscopy (SECM) has been combined with microfluidic devices to enable on-chip cell coculture and in situ investigation of the communication between triple-negative breast cancer cells (TNBCs) and TAMs in ferroptosis. In the coculture system, TNBCs and TAMs were used as responding cells and signaling cells, respectively. By in situ monitoring the changes of key parameters (ROS, glutathione (GSH), and cell membrane permeability) in Erastin-induced ferroptosis, it was found that TAMs partially restored the reduced GSH efflux, increased ROS release, and impaired cell membrane barrier in TNBCs, indicating that TAMs can suppress TNBC ferroptosis. Mechanistically, TNBCs could promote M2 macrophage polarization, and M2-TAMs achieved suppression of TNBCs ferroptosis through the STAT3-related signaling pathway. After inhibition of STAT3, increased ROS release and membrane permeability as well as decreased GSH efflux of TNBCs were in situ monitored by SECM, demonstrating the intercellular communication mechanism in ferroptosis. Therefore, this work provides a potential strategy of targeting TAMs for ferroptosis-based TNBC therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sensors
Chemical Engineering-Bioengineering
CiteScore
14.50
自引率
3.40%
发文量
372
期刊介绍:
ACS Sensors is a peer-reviewed research journal that focuses on the dissemination of new and original knowledge in the field of sensor science, particularly those that selectively sense chemical or biological species or processes. The journal covers a broad range of topics, including but not limited to biosensors, chemical sensors, gas sensors, intracellular sensors, single molecule sensors, cell chips, and microfluidic devices. It aims to publish articles that address conceptual advances in sensing technology applicable to various types of analytes or application papers that report on the use of existing sensing concepts in new ways or for new analytes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


