不对称Hofmann-Löffler-Freytag-type反应通过瞬态碳离子络合物合并有机催化和光催化
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由于碳离子作为中间体具有不可缺少的作用,它一直是有机合成中的重要组成部分。然而,它们固有的不稳定性和显著的反应性给实现对映体控制带来了显著的挑战。使用普遍存在的C(sp3) -H键作为前体来实现高度对映选择性转化是一种非常重要的方法,但探索较少。在这里,我们详细介绍了在可见光照射下的不对称Hofmann-Löffler-Freytag-type反应,从而产生具有优异对映选择性的手性埃文斯助剂。该方案的特点是其高效率(周转频率,154 h−1)和广泛的官能团耐受性。机理研究表明,现有的双恶唑啉催化剂可以通过氢键效应加速反应,并通过与碳离子中间体的瞬态配位调节对映选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
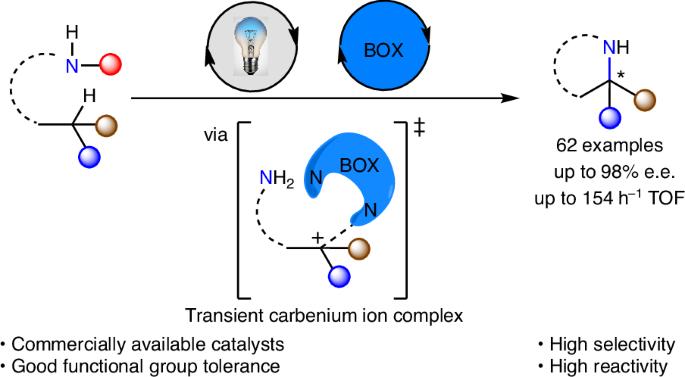

Asymmetric Hofmann–Löffler–Freytag-type reaction via a transient carbenium ion complex merging organocatalysis and photocatalysis
Carbenium ions have long been an important component in organic synthesis due to their indispensable role as intermediates. However, their inherent instability and remarkable reactivity pose notable challenges in achieving enantiocontrol. The use of ubiquitous C(sp3)–H bonds as precursors to achieve highly enantioselective transformations is a very important, yet less explored approach. Here we detail an asymmetric Hofmann–Löffler–Freytag-type reaction under visible-light irradiation, resulting in chiral Evans auxiliaries with excellent enantioselectivity. This protocol is distinguished by its high efficiency (turnover frequency, 154 h−1) and broad functional group tolerance. Mechanistic investigations reveal that the readily available bisoxazoline catalysts can expedite the reaction through a hydrogen-bonding effect and regulate enantioselectivity by transient coordination with carbenium ion intermediates. Enantiocontrolled transformation of carbenium ions is challenging due to their instability and high reactivity. Now, combining a chiral organocatalyst with a photocatalyst enables enantioselective intramolecular amidation of C(sp3)–H bonds to afford chiral oxazolidine products via a transient carbenium ion complex.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


