TEMPO阳离子和NaClO2介导的oxa -铁离子重排反应:在全合成钝化内酯B和C中的应用
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
TEMPO+(2,2,6,6-四甲基哌啶- n -氧基阳离子)是一种多用途的化学物质,通常被称为氧化试剂。然而,它作为路易斯酸的能力最近已经被揭示出来。在这里,我们报道了TEMPO+促进的oxa-Ferrier将甘糖重排为手性α,β-不饱和δ-内酯,使用亚氯酸钠(NaClO2)作为廉价和环保的氧化试剂。由于乙烯基氧羰基中间体被亚氯酸盐离子捕获形成羰基,我们将此反应命名为“氧-铁重排”。因此,该反应适用于各种o -乙酰化、o -苯甲酰化和o -苯甲酰化的糖基,可得到相应的α,β-不饱和δ-内酯,产率中等至较高。此外,该方法的合成效用被应用于被动内酯B和C的绝对构型的合成和确认。本文章由计算机程序翻译,如有差异,请以英文原文为准。
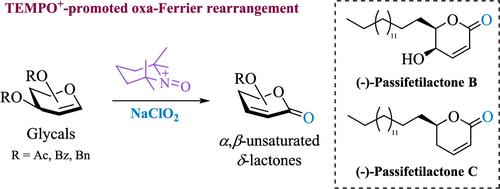
Oxa-Ferrier Rearrangement Reaction Mediated by TEMPO Cation and NaClO2: Application to the Total Synthesis of Passifetilactones B and C
The TEMPO+ (2,2,6,6-tetramethylpiperidine-N-oxyl cation) is a versatile chemical species commonly known as an oxidizing reagent. Nevertheless, its capability to act as a Lewis acid has been recently revealed. Here, we report a TEMPO+-promoted oxa-Ferrier rearrangement of glycals to chiral α,β-unsaturated δ-lactones using sodium chlorite (NaClO2) as a cheap and environmentally friendly oxidizing reagent. Since the vinylic oxocarbenium intermediate is trapped by chlorite ion to form a carbonyl group, we name this reaction as the “Oxa-Ferrier rearrangement”. Accordingly, this reaction is suitable for various O-acetylated, O-benzoylated, and O-benzylated glycals, providing the corresponding α,β-unsaturated δ-lactones in moderate to good yield. Additionally, the synthetic utility of this methodology was applied to the synthesis and confirmation of the absolute configuration of passifetilactones B and C.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


