镍催化芳香硼酸为芳基化试剂的三组分1,2-烷基芳基化反应
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
近年来,镍催化易得烯烃的对映选择性自由基接力三组分1,2-烷基芳化引起了人们的广泛关注。然而,在以往的报道中,仅使用芳基卤化物或芳基锌试剂作为芳基化试剂。随着我们对有机硼化学的持续兴趣,在这里,我们报告了这类反应的氧化还原中性方案,易于操作,空气和湿度稳定,并且市售的芳基硼酸作为芳基化试剂。芳基硼酸的高稳定性也带来了大问题,如转化慢、反应性低。为了克服这些问题,我们选择使用更富电子的biimazoline (BiIm)配体,并且我们能够开发出具有良好效率和对映选择性的4- cl - ph取代BiIm配体。通过我们的方法,已经证明了23种不同的芳硼酸和杂芳硼酸是有效的偶联伙伴,并且在温和的条件下,一些容易获得的烯烃以高收率和对映体过量的方式被双碳官能化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
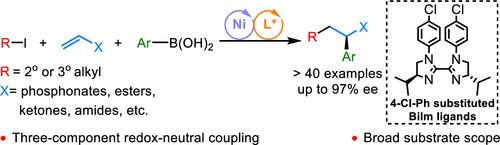
Nickel-Catalyzed Enantioselective Three-Component 1,2-Alkylarylation of Alkenes with Arylboronic Acids as Arylation Reagents
In recent years, Ni-catalyzed enantioselective radical relay three-component 1,2-alkylarylation of easily accessible alkenes has attracted significant attention. However, only aryl halides or aryl zinc reagents have been employed as arylation reagents in previous reports. With our continuous interest in organo-boron chemistry, herein, we report a redox-neutral protocol of this type of reaction, with easy-to-handle, air- and moisture-stable, and commercially available arylboronic acids as arylation reagents. The high stability of arylboronic acids also caused big problems, for example, slow transmetalation and low reactivity. To overcome these problems, we chose to use more electron-rich biimidazoline (BiIm) ligands, and we were able to develop a 4-Cl-Ph-substituted BiIm ligand, which gives good efficiency and enantioselectivity. Through our approach, 23 different arylboronic and heteroarylboronic acids have been demonstrated as efficient coupling partners, and a couple of easily accessible alkenes have been dicarbofunctionalized in good yields and enantiomeric excesses under mild conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


