氧化物的化学性质决定了铜|氧化物界面在电化学CO2还原反应中的稳定性和反应活性
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
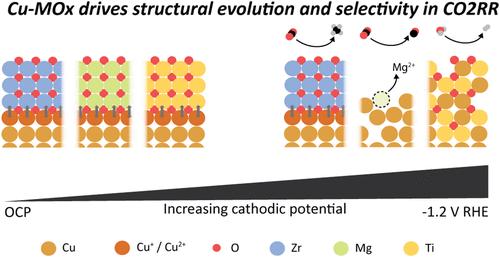
了解金属和氧化物之间的组成和界面的影响是许多化学反应感兴趣的目标。在此,我们提出了一个框架来绘制氧化物的电化学行为与金属|氧化物界面的稳定性和反应性之间的相关性,以Cu|氧化物为例,用于电化学CO2还原反应(CO2RR)。与金属氧化物界面的铜材料已成为有前途的CO2RR催化剂,可选择性地产生包括醇在内的多碳产物;据报道,其中一些在运行中情况稳定。然而,目前缺乏设计规则。在此,我们提议合成定义良好的Cu-MOx核壳纳米粒子,以研究和比较Cu-ZrOx, Cu-MgOx和Cu-TiOx的行为。通过跟踪这些模型催化剂材料的形态形成和形态演变,我们发现形成的界面的阴极稳定性是由纯氧化物的操作电位和相稳定性以及它们与铜的化学相互作用决定的。我们了解到这些因素之间的相互作用决定了Cu-MOx催化剂的重组途径,并最终驱动了它们在CO2RR中的选择性。所开发的纳米材料可以应用于催化以外的领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The Chemical Nature of the Oxide Directs the Stability and Reactivity of Copper|Oxide Interfaces in the Electrochemical CO2 Reduction Reaction
Understanding the impact of composition and interfaces between metals and oxides is a goal of interest for many chemical reactions. Herein, we propose a framework to map correlations between the electrochemical behavior of the oxide and the stability and reactivity of metal|oxide interfaces, exemplified by Cu|oxide for the electrochemical CO2 reduction reaction (CO2RR). Copper materials interfaced with metal oxides have emerged as promising CO2RR catalysts for selectivity toward multicarbon products, including alcohols; stability under operation has been reported for some of them. However, design rules are currently lacking. Herein, we propose the synthesis of well-defined Cu-MOx core–shell nanoparticles to investigate and compare the behavior of Cu-ZrOx, Cu-MgOx, and Cu-TiOx. By tracking the speciation and morphological evolution of these model catalyst materials, we find that the cathodic stability of the formed interfaces is determined by the operating potential and phase stability of the pure oxides and by their chemical interaction with copper. We learn that the interplay between these factors shapes the restructuring pathways for Cu-MOx catalysts and eventually drives their selectivity in the CO2RR. The developed understanding can be applied beyond this reaction, and the developed nanomaterials can be used beyond catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


