用化学循环的概念从高炉煤气中分离和净化CO2
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
低成本的钢铁工业二氧化碳分离净化技术也备受关注。在这里,我们建议使用赤泥(RM)作为氧载体(OC)用于高炉煤气(BFG)化学环燃烧(CLC),从而实现BFG转化的低成本二氧化碳捕集。结果表明,广西(GX) RM的CO2捕集率和转化率平均分别为97.6% %和99.5% %。活性组分(Fe2O3)和适当含量的惰性/碱性组分(Al2O3、Na2O、CaO和MgO)的各种相互作用增强了GX-RM的整体性能。OC高性能的另一个因素是Fe和Ti氧化物(Fe2TiO5)之间的协同效应。根据TPD、XPS和原位DRIFTS数据,导致CO2生成的主要过程是晶格氧与CO的直接反应,以及CO通过CO在OC表面吸附与活性氧的反应。热重动力学分析表明,CO-GX-RM反应的相边界控制(收缩柱)模型的活化能为29.18 kJ/mol。进一步研究GX-RM的物相和组成表明,CLC处理增加了元素的分散,同时破坏了RM的致密结构。这项工作建立了一种有效的从工业副产品气体中捕获二氧化碳的策略,并通过使用RM作为OC在BFG中捕获二氧化碳,建立了一种可持续的废物处理选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。

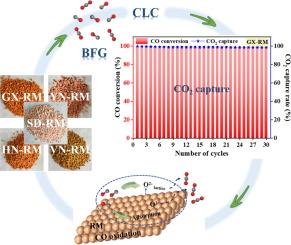
Separation and purification of CO2 from blast furnace gas combustion by a chemical looping concept
Low-cost technology for CO2 separation and purification from steel industry has also attracted much attention. Here, we propose using red mud (RM) as an oxygen carrier (OC) for blast furnace gas (BFG) chemical looping combustion (CLC), resulting in low-cost CO2 capture from BFG conversion. The results showed that Guangxi (GX) RM attained CO2 capture and conversion rates of 97.6 % and 99.5 % on average, respectively. The overall performance of the GX-RM is enhanced by various interactions of the active components (Fe2O3) and the appropriate content of the inert/alkaline components (Al2O3, Na2O, CaO, and MgO). An additional factor in the high performance of the OC is the synergistic effects between Fe and Ti oxides (Fe2TiO5). According to TPD, XPS, and in-situ DRIFTS data, the primary processes leading to the CO2 production were the direct reaction between lattice oxygen and CO, and the reaction of CO with active oxygen species through CO adsorption on the surface of OC. Kinetic analysis using a thermogravimetric method suggests that the phase-boundary-controlled (contracting cylinder) model could be a viable fit for the CO-GX-RM reaction, with an activation energy of 29.18 kJ/mol. Further research into the phases and compositions of GX-RM showed that the CLC treatment increased the dispersion of elements while destroying the dense structure of RM. This work has established an effective strategy for CO2 capture from industrial byproduct gas and a sustainable waste treatment option through the use of RM as OC to capture CO2 in BFG.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


