多尺度模拟引导电场增强氨催化裂化
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氨催化裂化为氢气的生产、储存和分配提供了有效的解决方案,当与燃料电池集成在一起时,它是船用推进系统中船用制氢的理想选择。然而,传统的加热方法,即使是高活性的钌(Ru)催化剂,也需要高温才能达到令人满意的性能,这对工业实施提出了挑战。解决这一限制的一个有希望的策略是应用强外电场,它可以通过在氨裂解过程中电场与极化物质的偶极子之间的相互作用来降低温度要求。为了探索这种场偶极子效应,我们开发了一个多尺度模拟框架,将密度泛函理论(DFT)计算与微动力学建模相结合。该框架提供了机理见解,确定了关键的限速步骤,并优化了Ru上现场增强氨催化裂化的条件。结果表明,在673 K时,施加−1 V/Å负电场可使翻转频率从0.03 s-1(零场)急剧增加到1435.2 s-1。同样,在823 K的较高温度下,负电场比无电场条件下,翻转频率提高了4个数量级。此外,施加−1 V/Å电场将工作温度从750 K(零场)降低到586k,同时保持给定的翻转频率(例如5 s-1)。灵敏度分析进一步确定Ru(1013)上的NH脱氢是在不同电场和温度下的限速步骤。这个多尺度模型增强了对现场增强催化的理解,为开发更高效的制氢工艺提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
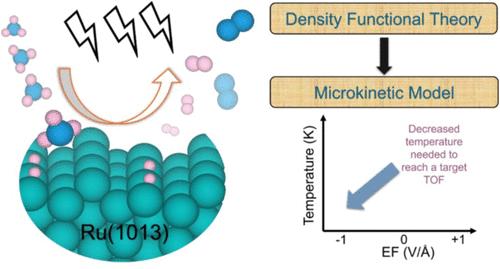
Multiscale Simulation Guided Electric Field-Enhanced Ammonia Catalytic Cracking
Ammonia catalytic cracking offers an efficient solution for hydrogen production, storage, and distribution, making it ideal for onboard hydrogen generation in maritime propulsion systems when integrated with fuel cells. However, conventional heating methods, even with highly active ruthenium (Ru) catalysts, require high temperatures to achieve satisfactory performance, posing a challenge for industrial implementation. A promising strategy to address this limitation is the application of strong external electric fields, which can lower the temperature requirement through interactions between fields and the dipoles of polarized species during ammonia cracking. To explore such a field-dipole effect, we developed a multiscale simulation framework that integrates density functional theory (DFT) calculations with microkinetic modeling. This framework provides mechanistic insights, identifies key rate-limiting steps, and optimizes conditions for field-enhanced ammonia catalytic cracking over Ru. Our results show that at 673 K, applying a −1 V/Å negative electric field dramatically increases the turnover frequency from 0.03 s–1 (zero field) to 1435.2 s–1. Similarly, at a higher temperature of 823 K, the negative electric field enhances the turnover frequency by 4 orders of magnitude compared to the no field conditions. In addition, applying a −1 V/Å electric field reduces the operating temperature from 750 K (zero field) to 586 K while maintaining a given turnover frequency (e.g., 5 s–1). Sensitivity analysis further identifies NH dehydrogenation over Ru(1013) as the rate-limiting step across various electric fields and temperatures. This multiscale model enhances the understanding of field-enhanced catalysis, offering valuable insights into the development of more efficient hydrogen production processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


