酸性介质中sn修饰Pd-Pt单晶电极电催化氧化亚氮还原反应
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
一氧化二氮(N2O)是一种温室气体和消耗臭氧层的气体。已知电催化N2O还原反应(N2ORR)是在钯、铂等贵金属电极上催化的,在酸性介质中,用锡对这些贵金属进行表面修饰可以提高N2ORR。然而,锡在地表的作用仍不清楚。在这项工作中,研究了在酸性介质中,在电极表面存在和不存在Sn的情况下,具有(111)或(100)平面的单晶Pt, Pd和Pd - Pt电极的N2ORR活性。对Sn修饰Pt(111)和Pd(111)电极的原位x射线晶体截断棒(CTR)测量显示,在其表面存在金属Sn和SnO。表面Sn修饰提高了Pd - Pt(100)和Pd(100)电极的N2ORR活性,但对Pt(111)、Pd - Pt(111)和Pt(100)电极没有作用。Sn修饰的15原子% Pd - Pt(100)电极的N2ORR活性高于Sn修饰的Pd(100)或Pt(100)电极,表明Pd和Pt在(100)表面与Sn的存在对N2ORR活性的最大化是重要的。密度泛函理论(DFT)计算表明,Sn/ Pd-Pt(100)电极的N2ORR活性可归因于(1)(100)表面较强的N2O吸附能力,(2)Pd-Pt合金化对H中毒的减少,(3)Sn修饰对N2O分子的活化。我们使用原子定义单晶电极的模型研究将使研究人员能够从表面工程的角度设计和开发实用的N2ORR电催化剂,然后通过去除N2O为减缓全球变暖和平流层臭氧保护做出贡献。本文章由计算机程序翻译,如有差异,请以英文原文为准。
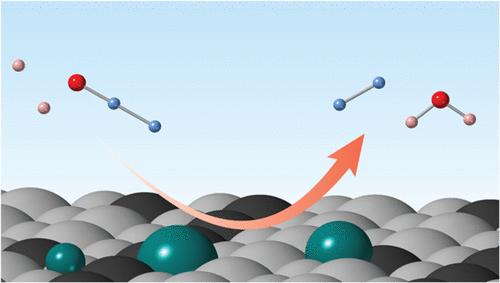
Electrocatalytic Nitrous Oxide Reduction Reaction at Sn-Modified Pd–Pt Single Crystalline Electrodes in Acidic Media
Nitrous oxide (N2O) is a greenhouse and an ozone-depleting gas. Electrocatalytic N2O reduction reaction (N2ORR) is known to be catalyzed at noble metal electrodes such as Pd and Pt, and the surface modification of such noble metals with Sn is known to increase the N2ORR in acidic media. However, the role of Sn at the surface remains unclear. In this work, N2ORR activity was investigated for single-crystalline Pt, Pd, and Pd–Pt electrodes with the (111) or (100) plane in the presence and absence of Sn at the electrode surface in acidic media. In situ X-ray crystal truncation rod (CTR) measurements of Sn-modified Pt(111) and Pd(111) electrodes revealed the presence of metallic Sn and SnO at their surfaces. The surface Sn modification enhances the N2ORR activity for Pd–Pt(100) or Pd(100) electrodes but not for the Pt(111), Pd–Pt(111), or Pt(100) electrodes. The Sn-modified 15 atom% Pd–Pt(100) electrode shows higher N2ORR activity than Sn-modified Pd(100) or Pt(100) electrodes, indicating that the copresence of Pd and Pt at the (100) surface with Sn is important to maximize the N2ORR activity. Density functional theory (DFT) calculations revealed that the N2ORR activity of the Sn/Pd–Pt(100) electrode can be attributed to (1) the strong N2O adsorption capacity of the (100) surface, (2) the reduction of H poisoning by Pd–Pt alloying, and (3) the activation of N2O molecules by Sn modification. Our model studies using atomically defined single-crystalline electrodes will enable researchers to design and develop practical N2ORR electrocatalysts from the surface engineering point of view and then contribute to global warming mitigation and stratospheric ozone protection through N2O removal.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


