1,3-二烯与光的可控自由基反应
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
原料化学品直接转化为增值产品是合成化学领域的一个热门主题。由于1,3-二烯是最普遍和最容易合成的起始材料,因此开发其功能化的合成方法非常有兴趣。然而,由于1,3-二烯上存在多个反应位点,实现高区域和对映体选择性仍然是难以实现的。1,3-二烯转化的传统合成工艺经常面临挑战,如低选择性,高能量消耗和产生不良副产物。在合成技术进步的推动下,利用自由基的合成方法已经成为创造有价值的化学基序的强大平台。光催化方法利用光能在温和条件下促进自由基反应,增强了对反应途径的控制,提高了选择性。在这篇综述中,我们对这些基于自由基的光催化创新技术在1,3-二烯的可控发散自由基反应中的应用进行了深刻的机理综述。反应分为1,2-和1,4-氢化反应和1,2-和1,4-双官能化反应。并对这一新兴领域的未来发展进行了展望。本文章由计算机程序翻译,如有差异,请以英文原文为准。
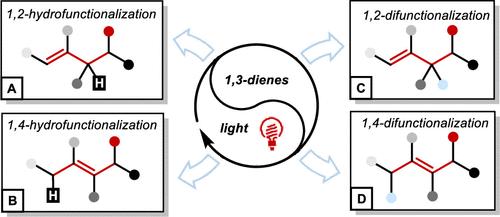
Controllable Radical Reactions of 1,3-Dienes with Light
The direct conversion of feedstock chemicals into value-added products is a popular theme in synthetic chemistry. Since 1,3-dienes are some of the most ubiquitous and readily synthesizable starting materials, there is great interest in developing synthetic methods for their functionalization. However, due to the existence of multiple reactive sites on the 1,3-diene, achieving high regio- and enantioselectivity remains elusive. Traditional synthetic processes for 1,3-diene transformations often face challenges, such as low selectivity, high energy consumption, and the generation of undesirable byproducts. Driven by advances in synthetic technologies, synthesis methods utilizing radicals have emerged as a powerful platform for creating valuable chemical motifs. Photocatalytic approaches harness light energy to facilitate radical reactions under mild conditions, offering enhanced control over the reaction pathways and improved selectivity. In this review, we provide an insightful mechanistic overview of these radical-based photocatalytic innovations used in the controllable divergent radical reactions of 1,3-dienes. The reactions are categorized into 1,2- and 1,4-hydrofunctionalization and 1,2- and 1,4-difunctionalization. Perspectives on the future development of this emerging field are also given.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


