骨靶向核酸传递聚合物载体有效治疗骨转移
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
骨病,如骨转移,由于骨组织不同的生理环境,给治疗带来了重大挑战,这使得核酸治疗的靶向递送复杂化。现有的输送系统,包括脂质纳米颗粒(LNP)和聚乙烯亚胺(PEI),难以有效地实现精确的骨靶向。为了解决这一问题,我们开发了一种基于聚合物的骨靶向生物可还原核酸递送载体,聚[阿仑膦酸-co-(N,N ' -双(丙烯酰)半胺-co-4-氨基-1-丁醇](ALN- pabol),其中含有阿仑膦酸(ALN)用于精确骨靶向。ALN-Pabol的羟基磷灰石结合率为91.1%,明显优于非靶向Pabol/miRNA(73.5%)和PEI/miRNA(58.3%)和Lipofectamine 2000/miRNA(64.7%)等商用系统。与非靶向对照相比,体内荧光成像显示其具有优越的骨骼积累。在小鼠乳腺癌骨转移模型中,ALN-Pabol/miRNA复合物与PBS对照相比,使骨肿瘤重量减少79.1%,与LNP/miRNA相比,使骨肿瘤重量减少36.8%。机制上,该复合物在高谷胱甘肽肿瘤微环境中解离,释放治疗性miRNA,抑制癌细胞增殖,促进细胞凋亡。同时,ALN抑制破骨细胞活性,显著减轻溶骨损伤。显微ct分析显示骨体积和小梁结构几乎完全恢复到健康水平。这项工作确立了ALN-Pabol作为骨靶向基因治疗的极具前景的传递载体,填补了骨骼疾病治疗的关键空白,并扩大了骨再生和癌症治疗的潜在应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
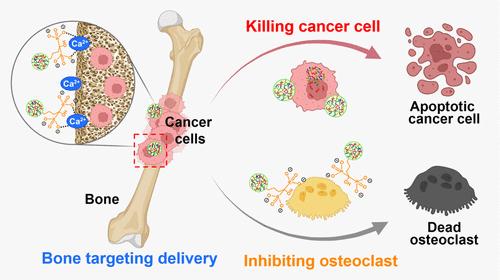
Bone-Targeting Nucleic Acid Delivery Polymer Vector for Effective Therapy of Bone Metastasis
Bone diseases, such as bone metastases, pose significant therapeutic challenges due to the distinct physiological environment of skeletal tissues, which complicates the targeted delivery of nucleic acid therapeutics. Existing delivery systems, including lipid nanoparticles (LNP) and polyethylenimine (PEI), struggle to achieve precise bone targeting effectively. To address this issue, we developed a polymer-based bone-targeting bioreducible nucleic acid delivery vector, poly[alendronic acid-co-(N,N′-bis(acryloyl)cystamine-co-4-amino-1-butanol)] (ALN-Pabol), which incorporates alendronic acid (ALN) for precise bone targeting. The ALN-Pabol exhibited a hydroxyapatite binding rate of 91.1%, significantly outperforming nontargeted Pabol/miRNA (73.5%) and commercial systems such as PEI/miRNA (58.3%) and Lipofectamine 2000/miRNA (64.7%). In vivo fluorescence imaging demonstrated its superior skeletal accumulation compared to nontargeted controls. In a murine breast cancer bone metastasis model, ALN-Pabol/miRNA polyplex reduced bone tumor weight by 79.1% relative to PBS controls and 36.8% compared to LNP/miRNA. Mechanistically, the polyplex dissociates in the high-glutathione tumor microenvironment, releasing therapeutic miRNA to suppress cancer cell proliferation and promote apoptosis. Simultaneously, ALN inhibits osteoclast activity, significantly mitigating osteolytic damage. Micro-CT analysis revealed near-complete restoration of bone volume and trabecular architecture to healthy levels. This work establishes ALN-Pabol as a highly promising delivery vector for bone-targeted gene therapy, bridging critical gaps in skeletal disease treatment and expanding potential applications in bone regeneration and cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


