利用光热增强等离子体传感系统进行热等离子体调节和生物分子的原位检测
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
等离子体近场无标记生物传感技术是一种很有前途的生物分子物质定量分析工具,可用于疾病诊断、病原体生物防御和环境监测。然而,对于复杂样品,等离子体分子传感的能力受到非特异性干扰的阻碍。近场热等离子体效应以局部表面等离子体共振(LSPR)的相互关联和协同现象为特征,赋予了等离子体生物传感潜在的多功能性。本文提出了光热增强等离子体(PTEP)传感系统,该系统实现了近场光热加热调节、原位温度监测、生物分子调节和等离子体界面平行生物传感。采用高时空分辨率的PTEP系统对均匀激光激发形成的光热近场进行了原位表征和热调节。值得注意的是,所提出的PTEP生物传感器系统由于热等离子体增强的折射率对比而表现出更高的灵敏度。此外,热等离子体场的精确时空规划有助于有效的防污和目标分子的特异性识别。基于PTEP生物传感器,提出了一种热等离子体生物传感策略,用于快速分析小鼠模型复杂脑脊液样品中的痕量IL-6分子,检测限低至0.1 pM。我们提出的PTEP生物传感方法提供了一种多功能和适应性强的策略,有可能增强纳米等离子体生物传感器的功能和实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
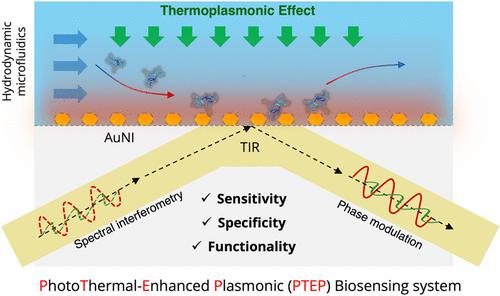
Thermoplasmonic Regulation and In Situ Detection of Biomolecules with a Photothermal-Enhanced Plasmonic Biosensing System
Label-free biosensing via plasmonic near-fields is a promising tool for quantitative analysis of biomolecular substances for disease diagnosis, pathogen biodefense, and environmental monitoring. For complex samples, however, the competence of molecular sensing with plasmonics is hampered by nonspecific interferences. The near-field thermoplasmonic effect, characterized by an interrelated and synergistic phenomenon of Localized Surface Plasmon Resonance (LSPR), empowers the potential multifunctionality of plasmonic biosensing. This work presented the photothermal-enhanced plasmonic (PTEP) sensing system, which enabled near-field photothermal heating regulation, in situ temperature monitoring, biomolecular regulation, and parallel biosensing at the plasmonic interface. The photothermal near-fields constructed through homogenized laser excitation were characterized and thermoregulated in situ by the PTEP system with a high spatiotemporal resolution. Notably, the proposed PTEP biosensor system exhibited improved sensitivity attributed to the thermoplasmonic-enhanced refractive index contrast. Moreover, precise spatiotemporal programming of the thermoplasmonic field contributed to active antifouling and specific identification of target molecules. Based on the PTEP biosensors, a thermoplasmonic biosensing strategy was proposed for rapid analysis of trace IL-6 molecules in complex cerebrospinal fluid samples from mouse models, with a detection limit down to 0.1 pM. Our proposed PTEP biosensing method offers a versatile and adaptable strategy that potentially enhances the functionality and utility of nanoplasmonic biosensors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


