钾离子电池中基于热绿石的插层阳极氧化还原电势的演变
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
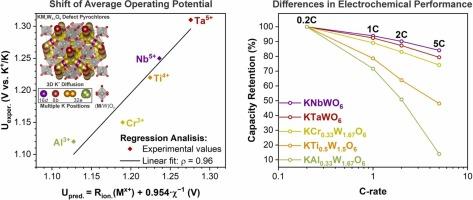
钨氧化物及相关化合物作为金属离子电池的负极材料已有几十年的历史。尽管其结构灵活,但大多数研究主要集中在锂基储能系统上。本文研究了采用缺陷焦绿盐结构的KMxW2−xO6 (M = Ta, Nb, Ti, Cr, Al)氧化物作为钾离子电池的插层型阳极。对所有代表材料的晶体结构、化学组成和热行为进行了全面表征。在K半电池中的电化学测试表明,所考虑的焦绿石相对于K+/K的平均工作电位在~1.1-1.3 V范围内,与密度泛函理论预测一致。KMxW2−xO6的电化学性能变化与M掺杂剂的电导率差异有关,M态密度计算证实了这一点。通过rietveld精细结构数据的回归分析,发现KMxW2−xO6的平均插层电位与M金属的本征参数(离子半径和电负性)之间存在很强的线性关系(ρ = 0.96)。本研究不仅促进了对钨基缺氧焦绿石的基本认识,而且为其作为钾离子插层寄主的发展铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The evolution of redox potentials in pyrochlore-based intercalation anodes for potassium-ion batteries
Tungsten oxides and related compounds have been known as negative electrode materials for metal-ion batteries for decades. Despite their structural flexibility, most studies has largely focused on lithium-based energy storage systems. Here, we investigated KMxW2−xO6 (M = Ta, Nb, Ti, Cr, Al) oxides adopting a defect pyrochlore structure as intercalation-type anodes for potassium-ion batteries. Crystal structure, chemical composition, and thermal behavior of all representatives were comprehensively characterized. Electrochemical testing in K half-cells revealed average operating potentials of the considered pyrochlores to be in the range of ∼1.1–1.3 V vs. K+/K, consistent with density functional theory predictions. The variation in the electrochemical performance among the KMxW2−xO6 was correlated with differences in electronic conductivity of M dopants, as validated by M density of states calculations. Through regression analysis of Rietveld-refined structural data, a strong linear dependence (ρ = 0.96) between the average intercalation potential of KMxW2−xO6 and the intrinsic parameters (ionic radius and electronegativity) of the M metal was established. This work not only advances fundamental understanding of tungsten-based oxygen-deficient pyrochlores but also paves the way for their development as potassium-ion intercalation hosts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


