胃粘液穿透性反应性微凝胶缓解幽门螺杆菌引起的胃炎
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
幽门螺杆菌(Helicobacter pylori, H. pylori)是一种普遍存在的全球性病原体,可导致胃炎和胃癌的潜在发展。萝卜硫素(SFN)是一种食源性化合物,对幽门螺杆菌具有显著的抗菌作用。然而,由于稳定性差和易受环境退化的影响,其效用受到限制。在这里,我们开发了一种胃反应释放微凝胶,用于输送抗h。螺杆菌SFN。通过交联α-乳清蛋白纳米管制备微凝胶,并包覆壳聚糖(cts - mg)。SFN以10.73 ± 0.25 %的加载率加载到微凝胶中。CTS-MG与胃粘膜黏附较强,可延长胃潴留时间达24 h,并在胃中反应性释放SFN。此外,CTS-MG/SFN解离并释放纳米管/SFN,该纳米管/SFN可以穿透胃黏液层,到达幽门螺杆菌聚集的粘液最深处。结果表明,CTS-MG/SFN对幽门螺杆菌具有明显的抑制作用。口服CTS-MG/SFN对幽门螺杆菌感染小鼠有效缓解幽门螺杆菌引起的胃炎,调节胃微生物群稳态。这项工作表明CTS-MG微凝胶在胃靶向和口服抗生素天然化合物治疗幽门螺杆菌感染方面具有很高的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。

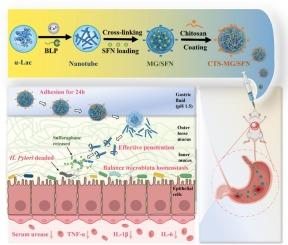
Gastric-mucus penetrating and responsive microgels for alleviating Helicobacter pylori-induced gastritis
Helicobacter pylori (H. pylori) is a prevalent global pathogen responsible for gastritis and the potential development of gastric cancer. Sulforaphane (SFN), a foodborne compound, exhibits notable antibiotic properties against H. pylori. However, its utility is limited by poor stability and susceptibility to environmental degradation. Here, we developed a gastric-responsive-release microgel for delivering anti-H. pylori SFN. The microgels were prepared by cross-linking α-lactalbumin nanotubes then coated with chitosan (CTS-MGs). SFN was loaded into microgels with a loading rate of 10.73 ± 0.25 %. The CTS-MG showed a strong adhesion to the gastric mucosa, prolonging gastric retention for up to 24 h and responsively releasing SFN in the stomach. Furthermore, CTS-MG/SFN dissociated and released nanotubes/SFN, which could penetrate into the gastric mucus layer and arrive at the deepest mucus sites where most H. pylori were colonized. Our results revealed that CTS-MG/SFN displayed an obvious inhibitory effect against H. pylori. The oral administration of CTS-MG/SFN in H. pylori-infected mice effectively alleviated H. pylori-induced gastritis and modulated gastric microbiota homeostasis. This work demonstrated high potential of CTS-MG microgels for gastric-targeted and oral delivery of antibiotic natural compounds against H. pylori infection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


