甲基丙烯酰水凝胶通过激活PEAK1-MAPK通路促进血管生成
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
由于现有治疗方法的局限性,口腔颌面部组织缺损的修复和再生仍然是一个重大挑战。传统的方法如移植、组织支架、生长因子和干细胞治疗经常面临障碍,包括供体短缺、血管化不足和安全问题。迫切需要创新的治疗策略来有效地促进血管再生,同时最大限度地减少并发症。黑磷纳米片(BPNSs)和水凝胶具有生物相容性、可降解性和药物控释特性,作为组织再生载体具有显著的优势和广阔的应用前景。结合各种表征技术和检测方法,我们对BPNSs和装载BPNSs的明胶甲基丙烯酰(GelMA)支架(BP-GelMA)进行了深入的分析。结果表明,本研究成功制备了尺寸均匀、分散性好、结构完整的BPNSs。此外,BP-GelMA复合材料表现出优异的溶胀性能和结构稳定性,同时有效地实现了BPNSs的可控释放。本实验研究了BP-GelMA在0、12.5和25.0 μg/mL浓度下的血管生成作用。体外实验表明,BP-GelMA可显著促进内皮细胞增殖、迁移和成管。体内实验结果表明,12.5 μg/mL和25.0 μg/mL BP-GelMA对斑马鱼没有明显的发育毒性,并能有效促进新生血管的形成。RNA-Seq分析显示BP-GelMA激活血管生成相关的生物学过程。机制研究发现PEAK1是一个中心调节因子,通过激活MAPK信号通路驱动血管形成。这些发现强调了BP-GelMA作为促进血管生成的治疗策略的潜力,并强调了优化BP-GelMA浓度以在临床应用中实现最大治疗效果和安全性的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
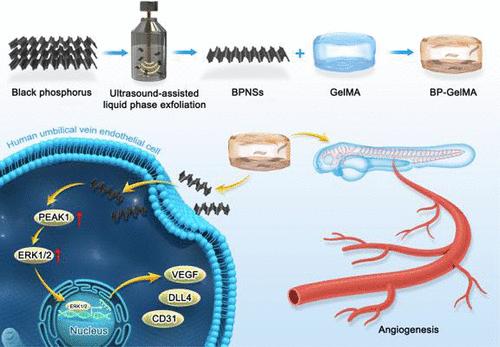
Black Phosphorus-Loaded Gelatin Methacryloyl Hydrogels Enhance Angiogenesis via Activation of the PEAK1–MAPK Pathway
Repair and regeneration of oral and maxillofacial tissue defects remain significant challenges, mainly due to the limitations of existing treatment approaches. Conventional methods such as transplantation, tissue scaffolds, growth factors, and stem cell therapies often face obstacles, including donor shortages, insufficient vascularization, and safety concerns. There is an urgent need for innovative therapeutic strategies to effectively promote vascular regeneration while minimizing complications. Black phosphorus nanosheets (BPNSs) and hydrogels present significant advantages and broad application potential as tissue regeneration carriers due to their biocompatibility, degradability, and controlled drug release properties. By combining various characterization techniques and detection methods, we conducted a thorough analysis of BPNSs and gelatin methacryloyl (GelMA) scaffolds loaded with BPNSs (BP-GelMA). The results indicate that this study successfully prepared BPNSs with uniform size, good dispersion, and intact structure. Moreover, the BP-GelMA composite demonstrated excellent swelling behavior and structural stability while effectively enabling the controlled release of BPNSs. This study investigated the angiogenic effects of BP-GelMA at concentrations of 0, 12.5, and 25.0 μg/mL. In vitro experiments showed that BP-GelMA significantly enhanced endothelial cell proliferation, migration, and tube formation. In vivo results demonstrated that 12.5 μg/mL and 25.0 μg/mL BP-GelMA did not induce significant developmental toxicity in zebrafish and effectively promoted neovascularization. RNA-Seq analysis revealed that BP-GelMA activates angiogenesis-related biological processes. Mechanistic studies identified PEAK1 as a central regulator, driving vascular formation through activation of the MAPK signaling pathway. These findings highlight the potential of BP-GelMA as a therapeutic strategy for promoting angiogenesis and underscore the importance of optimizing BP-GelMA concentrations to achieve maximum therapeutic efficacy and safety in clinical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


