光驱动水氧化动力学中空穴输运和热反应对TiO2晶体的影响
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
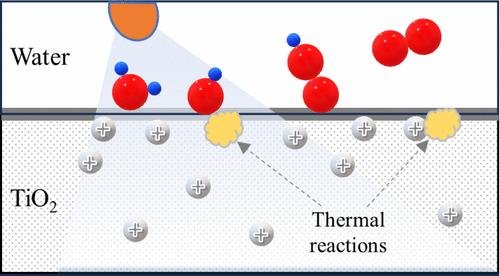
光生成的空穴累积驱动限速步骤的要求被认为导致TiO2缓慢的水氧化形成O2;然而,直接建立光吸收与表面反应之间联系的详细动力学研究尚未见报道。在这项工作中,我们使用物理上真实的光驱动水在TiO2上氧化的动力学模型来评估空穴产生、体扩散、表面迁移率和反应是如何耦合的。计算结果表明,在弱光强下,体晶中的空穴形成和扩散主导O2的形成,导致O2的生成速率明显高阶依赖于空穴。随着光强的增加,水分解反应几乎与空穴浓度无关,因为中间产物的积累只能进行热反应。虽然人们认为,高空穴迁移率是空穴积累的必要条件,但将预测的表面物质与观测到的表面物质进行比较表明,固定空穴主导了表面反应性。预计主要的表面反应位点涉及架桥两个Ti原子的氧原子,并在Ti位点上提供由水解离形成的OH。由于不同金属氧化物半导体的光催化水氧化机制具有相似性,通常具有低空穴迁移率,因此本工作的发现也可能与它们相关。如果是这样的话,控制空穴迁移率和加速热步骤速率可能为提高水氧化效率提供了一条通用途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Influence of Hole Transport and Thermal Reactions in Photo-Driven Water Oxidation Kinetics on Crystalline TiO2
The requirement that photogenerated holes accumulate to drive the rate-limiting step is thought to cause slow water oxidation by TiO2 to form O2; however, detailed kinetic studies that directly establish the connection between photoabsorption and surface reactions have not been reported. In this work, we use physically realistic kinetic models of photo-driven water oxidation on TiO2 to evaluate how hole generation, bulk diffusion, surface mobility, and reaction are coupled. The calculations show that hole formation and diffusion in the bulk crystal dominate O2 formation at low light intensity, resulting in an apparent high-order dependence of the O2 production rate on holes. As the light intensity increases, the water-splitting reaction becomes nearly independent of hole concentrations because of a buildup of intermediates that can only react thermally. Although it is believed that high hole mobility is a requirement for hole accumulation, a comparison of predicted to observed surface species indicates that immobilized holes dominate the surface reactivity. The primary surface reaction sites are predicted to involve oxygen atoms that bridge two Ti atoms, supplied with OH formed by water dissociation on the Ti sites. Because of the similarity among photocatalytic water oxidation mechanisms on diverse metal oxide semiconductors, which generally have low hole mobilities, the findings from this work may be relevant to them as well. If so, manipulations of hole mobility and acceleration of the rate of thermal steps may provide a general pathway for improving water oxidation efficiency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


