有机金属聚合物在Ag(111)上的异构化:揭示分子间氢转移机制
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
脱卤化反应在表面合成中起着至关重要的作用,但脱卤化反应中的成键位点有时会与原来的卤素取代位点不同,从而导致意想不到的产物。揭示其机制对于低维纳米材料的原子精确制造至关重要,尽管它仍然难以捉摸。在此,我们报道了由Ag(111)脱溴衍生的有机金属聚合物的异构化,并通过结合扫描隧道显微镜、非接触原子力显微镜和密度泛函理论计算阐明了涉及分子间氢转移的机制。在室温下,前体1,4-二(3-溴噻吩-2-基)苯在Ag(111)上发生表面辅助脱溴反应,形成两种有机金属聚合物,其中成键位点对应于原始脱溴位点。当退火至393 K时,金属有机聚合物的异构化生成线性金属有机聚合物,其中成键位点与原始脱溴位点不匹配。对照实验与理论计算相结合表明,意想不到的异构化过程是通过聚合物链解离成表面稳定的双自由基单体或低聚物、分子间氢转移以及表面稳定的自由基与银原子的最终重组进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
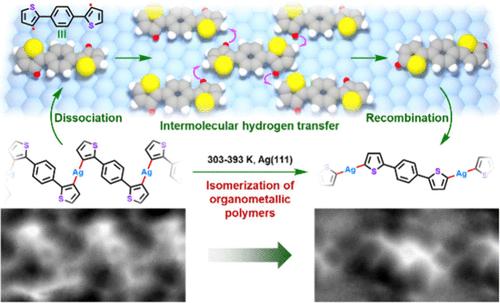
Isomerization of Organometallic Polymers on Ag(111): Revealing the Intermolecular Hydrogen Transfer Mechanism
Dehalogenation plays a crucial role in on-surface synthesis, but the bond-forming sites in dehalogenation occasionally differ from the original halogen-substituted sites, leading to unexpected products. Revealing its mechanism is essential for the atomically precise fabrication of low-dimensional nanomaterials, although it remains elusive. Herein, we report an isomerization of organometallic polymers derived from debromination on Ag(111) and elucidate the mechanism involving intermolecular hydrogen transfer via combining scanning tunneling microscopy, noncontact atomic force microscopy, and density functional theory calculations. At room temperature, the precursor 1,4-bis(3-bromothiophen-2-yl)benzene undergoes surface-assisted debromination on Ag(111), forming two organometallic polymers where the bond-forming sites correspond to the original debromination sites. Upon annealing to 393 K, the isomerization of organometallic polymers generates a linear organometallic polymer, where the bond-forming sites mismatched with the original debromination sites. Control experiments combined with theoretical calculations demonstrate that the unexpected isomerization proceeds through the dissociation of polymer chains into surface-stabilized diradical monomers or oligomers, intermolecular hydrogen transfer, and the final recombination of surface-stabilized radicals with Ag adatoms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


