用吡喃基聚合物调节高面积容量低温耐用镁金属电池的界面溶剂化环境
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
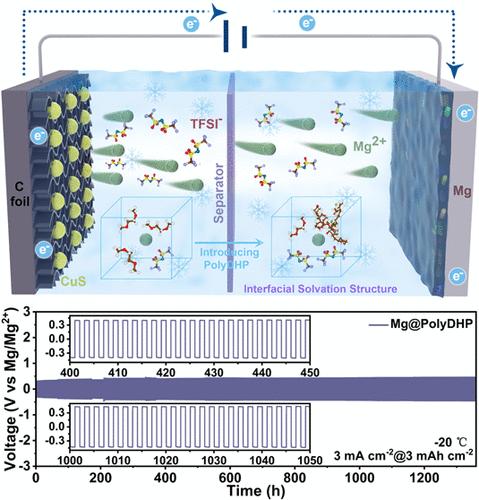
调控人造固体电解质界面相(SEI)和电解质的界面溶剂化结构对于开发具有长循环寿命、高电流密度耐受性和低温等极端环境下快速离子传输能力的可充电镁电池(RMBs)至关重要。本文提出了一种利用低聚聚(3,4-二氢- 2h -吡喃)(polyDHP)调节RMBs界面溶剂化结构的有效策略,即构建具有快速mg离子电导率的人工SEI。polyDHP的位阻及其与Mg2+的静电相互作用使第一溶剂壳中的溶剂分子减少,使polyDHP分子参与配位,从而降低了Mg2+的脱溶能垒,有利于其沉积和剥离。此外,由于玻璃化转变行为,低聚聚dhp在- 20℃时表现出更有序的结构和更连续的内部离子传输通道,从而首次实现了在较低温度下稳定的RMB操作。相应的Mg对称电池在室温(5 mA cm-2和10 mA h cm-2下超过5000小时)和- 20°C低温(3 mA cm-2和3 mA h cm-2下超过1300小时)下都显示出低得多的过电位(400 mV)和出色的循环稳定性。该策略支持在−20°C下稳定循环cu∥Mg满电池200次以上。这项工作揭示了调节界面溶剂化结构,促进极端条件下人民币实际应用的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Regulating the Interfacial Solvation Environment by a Pyran-Based Polymer for High-Areal-Capacity and Low-Temperature-Endurable Magnesium Metal Batteries
Regulating the artificial solid electrolyte interphase (SEI) and interfacial solvation structure of the electrolyte is crucial for developing rechargeable magnesium batteries (RMBs) with long cycling life, high current density tolerance, and fast ion transport capability operated under extreme environments, such as low temperatures. Herein, an effective strategy using oligomeric poly(3,4-dihydro-2H-pyran) (polyDHP) is proposed to modulate the interfacial solvation structure of RMBs, with the construction of an artificial SEI with rapid Mg-ion conductivity. The steric hindrance of polyDHP and its electrostatic interaction with Mg2+ reduce the solvent molecules in the first solvation shell, allowing polyDHP molecules to participate in coordination, thus lowering the desolvation energy barrier of Mg2+ and facilitating their deposition and stripping. Furthermore, due to the glass transition behavior, oligomeric polyDHP exhibits a more ordered structure with more continuous internal ion transport channels at −20 °C, therefore enabling stable RMB operation at lower temperatures for the first time. The corresponding Mg symmetric cells display a much lower overpotential (400 mV) and excellent cycling stability at both room temperature (over 5000 h at 5 mA cm–2 and 10 mA h cm–2) and a low temperature of −20 °C (over 1300 h at 3 mA cm–2 and 3 mA h cm–2). This strategy supports the stable cycling of CuS∥Mg full cells for over 200 cycles at −20 °C. This work reveals the importance of regulating the interfacial solvation structure, promoting the realistic applications of RMBs under extreme conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


