CuCo2S4/ g-C3N4-x S-Scheme异质结光热辅助光催化CO2还原
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
光催化将二氧化碳转化为化学燃料已成为研究热点,其目的是缓解化石燃料的快速枯竭和全球变暖问题。然而,光催化剂固有的低载流子分离效率和有限的太阳光利用率导致二氧化碳转化效率不尽人意。本研究采用简单的多元醇回流法成功制备了一种极具吸引力的 CuCo2S4/g-C3N4-x S 型异质结构。值得注意的是,氮空位增强了 CuCo2S4 和 g-C3N4-x 之间的费米级差,从而产生了更强的界面内置电场。全谱强光吸收能力赋予了合成催化剂卓越的光收集特性。光热效应引起的温度升高加速了催化剂表面 CO2 吸附和 CO 解吸的循环过程。最重要的是,S 型电荷转移途径确保了光生载流子的有效分离。得益于这些协同优势,CuCo2S4/g-C3N4-x 表现出卓越的光热辅助光催化二氧化碳还原性能。在模拟阳光下,CuCo2S4/g-C3N4-x 的平均 CO 生成率达到 24.64 μmol g-1 h-1,分别是 g-C3N4 和 CuCo2S4 的 12.1 倍和 27.1 倍。这项研究为设计具有优异二氧化碳转化性能的光催化剂提供了一种新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
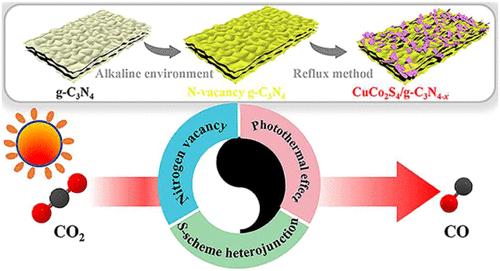
CuCo2S4/g-C3N4–x S-Scheme Heterojunction for Photothermal-Assisted Photocatalytic CO2 Reduction
Photocatalytic conversion of CO2 into chemical fuels has emerged as a research hotspot, aiming to mitigate the rapid depletion of fossil fuels and alleviate global warming. However, the inherent low carrier separation efficiency and limited solar light utilization of photocatalysts lead to unsatisfactory CO2 conversion efficiency. In this study, an appealing CuCo2S4/g-C3N4–x S-scheme heterostructure is successfully fabricated by a simple polyol reflux method. Notably, nitrogen vacancies enhance the Fermi level difference between CuCo2S4 and g-C3N4–x, resulting in a stronger interfacial built-in electric field. The full-spectrum strong optical absorption capability endows the synthesized catalysts with superior light-harvesting property. The photothermal effect-induced temperature increase accelerates the cyclic process of CO2 adsorption and CO desorption on the catalyst surface. Most importantly, the S-scheme charge transfer pathway ensures the efficient separation of photogenerated carriers. Thanks to these synergistic benefits, CuCo2S4/g-C3N4–x exhibits exceptional photothermal-assisted photocatalytic CO2 reduction performance. Under simulated sunlight, the average CO production rate of CuCo2S4/g-C3N4–x reaches 24.64 μmol g–1 h–1, which is 12.1 and 27.1 times higher than that of g-C3N4 and CuCo2S4, respectively. This study offers a novel strategy for designing photocatalysts with outstanding CO2 conversion performance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


