含pcp型钳形配体-茂金属钼配合物催化固氮生成亚胺的理论研究
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
均相催化剂使用单核氮化钼(Mo≡N)配合物承载pcp型钳形配体,允许在非常温和的条件下固氮。催化循环包括三个加氢过程,由mo -氮化物配合物[MoI(N)(PCP)]生成mo -胺配合物[MoI(NH3)(PCP)]。我们主要关注第一个加氢步骤,即形成mo -亚胺配合物[MoI(NH)(PCP)],因为之前的实验和理论研究表明,亚胺的形成是催化循环中的限速步骤。质子化剂和还原剂的选择对亚胺生成的催化活性有很大影响。在计算量子化学研究中,2,4,6-碰撞鎓(ColH+)作为质子化剂,金属茂物Cp2MII和十甲基金属茂物Cp*2MII (M = V, Cr, Mn, Fe, Co, Ni)作为还原剂。ColH+与茂金属反应生成质子化的茂金属,其中茂金属的环戊二烯环被质子化。质子化Cp*2CrII和Cp*2CoII是促进低激活自由能[MoI(N)(PCP)]亚胺形成的潜在质子耦合电子转移(PCET)介质。将协同反应机理与ColH+直接质子化[MoI(N)(PCP)],然后与十甲基茂金属还原的分步反应进行了比较。此外,我们还分析了质子转移和电子转移是如何在PCET介质与[MoI(N)(PCP)]的反应中协调一致的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
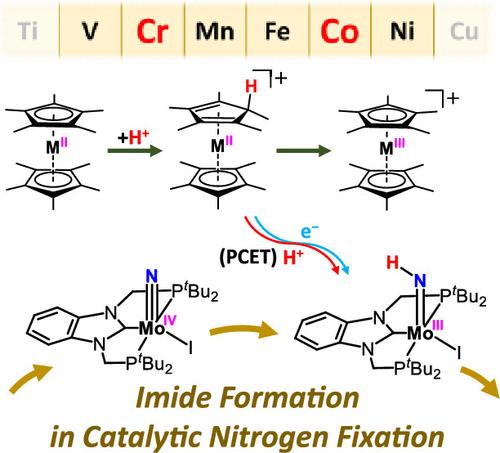
Theoretical Study of Imide Formation in Nitrogen Fixation Catalyzed by Molybdenum Complex Bearing PCP-Type Pincer Ligand with Metallocenes
Homogeneous catalysts using a mononuclear molybdenum nitride (Mo≡N) complex bearing PCP-type pincer ligands allow nitrogen fixation under very mild conditions. The catalytic cycle involves three hydrogenation processes yielding an Mo-ammine complex [MoI(NH3)(PCP)] from the Mo-nitride complex [MoI(N)(PCP)]. We primarily focused on the first hydrogenation step, forming an Mo-imide complex [MoI(NH)(PCP)] since previous experimental and theoretical studies suggest that imide formation is the rate-limiting step in the catalytic cycle. The choice of protonating agent and reductant strongly influences the catalytic reactivity in imide formation. In this computational quantum chemical study, 2,4,6-collidinium (ColH+) was employed as the protonation agent, while metallocenes Cp2MII and decamethylmetallocenes Cp*2MII (M = V, Cr, Mn, Fe, Co, and Ni) were employed as reductants. The reaction of ColH+ with the metallocenes yields protonated metallocenes, where a cyclopentadienyl ring of the metallocenes is protonated. Protonated Cp*2CrII and Cp*2CoII are potential proton-coupled electron transfer (PCET) mediators to facilitate the imide formation of [MoI(N)(PCP)] with low activation free energies. The concerted reaction mechanism was compared with the stepwise reaction, where ColH+ directly protonates [MoI(N)(PCP)], followed by reduction with the decamethylmetallocenes. Furthermore, we analyzed how proton transfer and electron transfer are concerted in the reaction of the PCET mediators with [MoI(N)(PCP)] by tracing electronic states along the reaction coordinates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


