利用固定化铜Salophen配合物电化学合成吩那嗪
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
本研究介绍了一种利用修饰电极Cu-PDABA-CFP作为电化学合成非那嗪的关键催化剂的新方法。通过在乙腈介质中大量电解邻苯二胺,高氯酸锂作为支撑电解质促进,电极作为合成努力的基本组成部分。修饰过程需要将铜-salophen复合物固定在电极表面,根据既定的文献协议合成。对修饰电极进行了细致的表面表征,以获得对电极表面结构形态和地形特征的关键见解,这对于理解其电化学行为至关重要。同时,进行了电化学表征研究,以评估修饰电极的固有活性。为了阐明合成转化背后复杂的电化学反应机制,对反应条件进行了详尽的筛选。本文提出的研究结果不仅有助于提高我们对电化学过程的基本理解,而且为在合成化学和材料科学中具有更广泛适用性的新型电化学方法的发展带来了希望。本文章由计算机程序翻译,如有差异,请以英文原文为准。

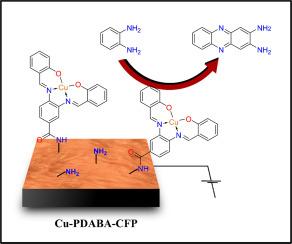
Harnessing immobilized copper salophen complex for the electrochemical synthesis of phenazine
This study introduces a novel approach utilizing a modified electrode, denoted as Cu-PDABA-CFP, as a pivotal catalyst in the electrochemical synthesis of phenazine. Through bulk electrolysis of o-phenylene diamine in an acetonitrile medium, facilitated by lithium perchlorate as the supporting electrolyte, the electrode serves as a fundamental component in this synthetic endeavor. The modification process entails the immobilization of a copper-salophen complex, synthesized in accordance with established literature protocols, onto the electrode surface. Surface characterization of the modified electrode was meticulously conducted to get critical insights into the structural morphology and topographical features of the electrode surface, pivotal for understanding its electrochemical behavior. Concurrently, electrochemical characterization studies were undertaken to evaluate the inherent activity of the modified electrode. To elucidate the intricate electrochemical reaction mechanism underlying the synthetic transformation, an exhaustive screening of reaction conditions was meticulously undertaken. The findings presented herein contribute not only to advancing our fundamental understanding of electrochemical processes but also hold promise for the development of novel electrochemical methodologies with broader applicability in synthetic chemistry and materials science.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


