天然有机物和二价阳离子在松散纳滤膜上/内的吸附:从排斥选择性的角度看对饮用水处理的影响
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
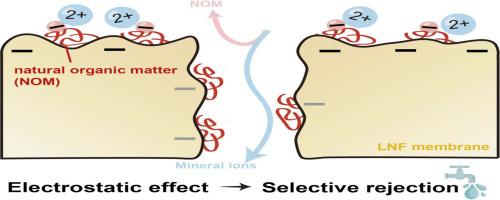
松散纳滤(LNF)膜在选择性过滤天然有机物(NOM)的同时保持矿物盐以生产高质量饮用水方面具有很大的前景。然而,膜的过滤选择性不仅取决于膜本身的特性,还受进水成分的影响。本研究探讨了NOM和无机离子不可避免地吸附在膜材料上和膜材料内部,从而改变LNF膜的电荷性质,从而影响其排斥选择性。采用Zeta电位测量、x射线光电子能谱和飞行时间二次离子质谱技术对膜表面和膜孔内溶质吸附进行了表征。通过合成水和天然水的过滤实验,考察了静电效应对膜的贡献,并对膜的截留性能进行了评价。结果表明,过滤过程中LNF膜表面很容易被NOM分子包裹,这可能是通过疏水相互作用,从而吸附了二价阳离子,而二价阳离子实际上决定了膜表面的净电荷密度。此外,膜孔内的NOM吸附在很大程度上改变了孔隙电荷性质,特别是磺化聚醚砜膜(例如NTR7450),其中去质子化的磺酸基有助于高电荷密度。NOM、二价阳离子和膜材料之间的相互作用大大降低了膜表面的电荷密度,大大减少了孔隙中的电荷,导致NOM和无机盐的排斥减少,并减轻了co-ion竞争效应。然而,UA60膜的分子量截止值为~ 1,000 Da,在处理水中保留~ 95%的碳酸氢盐和~ 65%的硬度离子的同时,拒绝了~ 70%的NOM,表现出相当好的选择性。这些发现为优化LNF膜以提高处理后饮用水的安全性、化学稳定性和适口性提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Adsorption of natural organic matter and divalent cations onto / inside loose nanofiltration membranes: Implications for drinking water treatment from rejection selectivity perspective
Loose nanofiltration (LNF) membranes hold great promise for the selective rejection of natural organic matter (NOM) while maintaining mineral salts to produce high-quality drinking water. Nevertheless, the rejection selectivity performance is not only determined by the inherent properties of membranes but also influenced by the feed water compositions. This study explored the inevitable adsorption of NOM and inorganic ions onto and inside membrane materials, which in turn altered the charge properties of LNF membranes, thereby affecting the rejection selectivity. Zeta potential measurements, X-ray photoelectron spectroscopy and time-of-flight secondary ion mass spectrometry technique were employed to characterize solute adsorption on the membrane surface and within the membrane pores. Filtration experiments using synthetic and natural waters were conducted to assess the contribution of electrostatic effects and evaluate the membrane rejection performance. Results revealed that LNF membrane surfaces during filtration were readily coated by NOM molecules, probably via hydrophobic interactions, which in turn adsorbed divalent cations that actually determined the net charge density on the membrane surface. Additionally, NOM adsorption within the membrane pores largely altered pore charge properties, particularly of the sulfonated polyethersulfone membranes (e.g. NTR7450), where deprotonated sulfonic groups otherwise contributed to a high charge density. These interactions among NOM, divalent cations and membrane materials greatly reduced charge density on the membrane surface and largely diminished charges in pores, leading to decreased rejection of both NOM and mineral salts, as well as the mitigation of co-ion competition effects. Nevertheless, the UA60 membrane, having a molecular weight cut-off of ∼1000 Da, rejected NOM by ∼70 % while maintaining ∼95 % bicarbonate and ∼65 % hardness ions in the treated water, demonstrating fairly good selectivity. These findings offer valuable insights for optimizing LNF membranes to improve the safety, chemical stability and palatability of treated drinking water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


