电子金属-载体相互作用中的邻近效应:Ru/ZrO2模型催化剂上o空位形成和CO吸附
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
继续研究电子金属-载体相互作用(emsi)在非均相催化中的作用,我们研究了空位位置和空位数量对Ru纳米棒稳定它们的影响,对从载体到金属的电荷转移的影响,以及对CO在Ru纳米棒上吸附的影响。通过基于密度泛函理论的计算,并使用由ZrO2(111)支架和三层Ru纳米棒组成的模型系统,我们发现o -空位只有在与Ru纳米棒直接接触时才会显着稳定,其稳定程度取决于空位与界面上最近的Ru原子之间的距离。在钌纳米棒旁边或在支架的更深层中,金属不会增强空位的形成。钌诱导的o -空位稳定与空位形成时从载体到金属的电荷转移密切相关,在邻近o -空位存在时也是如此。CO的吸附能可以通过四种特征效应得到很大的改变,包括从载体到金属的电荷转移、配位效应、CO诱导的变形能和界面能变化的组合以及CO与直接邻近o空位的部分还原Zr表面离子之间的直接相互作用,这取决于吸附位置和o空位的数量和位置。因此,用d波段模型不可能完全描述吸附性质,特别是不能描述界面位置上的吸附。讨论了这些发现与吸附和催化反应的一般相关性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
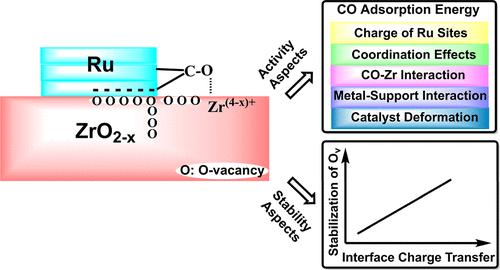
Proximity Effects in Electronic Metal–Support Interactions: O-Vacancy Formation and CO Adsorption on Ru/ZrO2 Model Catalysts
Continuing our investigation of electronic metal–support interactions (EMSIs) in heterogeneous catalysis, we have investigated the influence of the position and the number of O-vacancies on their stabilization by the Ru nanorod, on the charge transfer from the support to the metal, and on CO adsorption on the Ru nanorod. Employing density functional theory-based calculations and using a model system consisting of a ZrO2(111) support and a three-layer Ru nanorod, we find that O-vacancies are significantly stabilized only if they are in direct contact with the Ru nanorod, with the extent of stabilization depending on the distance between vacancy and the nearest Ru atom at the interface. Vacancy formation beside the Ru nanorod or in deeper layers of the support is not enhanced by the metal. The Ru-induced stabilization of the O-vacancies is closely coupled with the charge transfer from the support to the metal upon vacancy formation, which is true also in the presence of neighboring O-vacancies. The CO adsorption energy can be substantially modified by four characteristic effects, including charge transfer from the support to the metal, coordination effects, a combination of COad-induced deformation energies and changes in the interface energy and direct interactions between CO and partly reduced Zr surface ions directly neighboring to an O-vacancy, depending on the adsorption site and on the number and positions of the O-vacancies. Thus, it is not possible to completely describe the adsorption properties by using the d-band model, in particular, not for adsorption on the interface sites. The general relevance of these findings for adsorption and catalytic reactions is discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


