NIR-II光敏剂纳米颗粒通过促进热休克蛋白40使线粒体失功能克服肿瘤自卫
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
恶性肿瘤内固有的自我防御途径包括热休克蛋白(HSPs)的作用,并经常阻碍光热治疗的效果。有趣的是,HSP40抑制糖酵解并破坏线粒体功能以克服肿瘤自卫机制并表现出肿瘤抑制作用。i型光动力疗法产生的活性氧(ROS),尤其是羟基自由基,抑制了三磷酸腺苷(ATP)的产生,并导致热应激期间ATP无关的HSP40过表达。然而,高温和羟基自由基诱导HSP40表达的调控机制尚不清楚。因此,有必要阐明热应激诱导HSP40表达的潜在机制,并探索其作为抗肿瘤发展的潜在治疗策略。通过战略性地修改aza-BODIPY结构来精确地分配激发态能量,我们已经证明HSP40的特异性表达与羟基自由基的热量比例相关,而不是与它们的个体水平相关。这种基于NIR-II光敏剂的纳米颗粒减少了肿瘤糖酵解,破坏了ATP的产生,促进了细胞凋亡,增强了光热治疗的效果。热休克蛋白在高温和活性氧胁迫下的沉默和补偿是克服肿瘤自我防御机制的一种有前途和有效的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
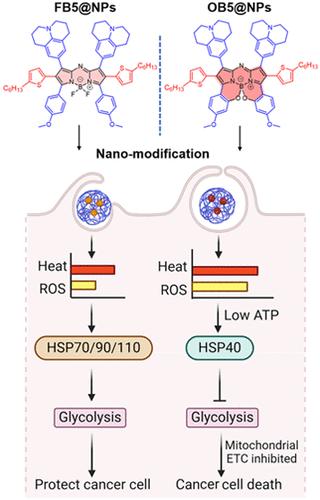
NIR-II Photosensitizer-Based Nanoparticles Defunctionalizing Mitochondria to Overcome Tumor Self-Defense by Promoting Heat Shock Protein 40
Inherent self-defense pathways within malignant tumors include the action of heat shock proteins (HSPs) and often impede photothermal therapy efficacy. Interestingly, HSP40 inhibits glycolysis and disrupts mitochondrial function to overcome tumor self-defense mechanisms and exhibits a tumor-suppressive effect. Reactive oxygen species (ROS), especially hydroxyl radicals, generated by type-I photodynamic therapy inhibit adenosine triphosphate (ATP) production and lead to ATP-independent HSP40 overexpression during heat stress. However, the regulatory mechanisms linking heat and hydroxyl radicals to induce HSP40 expression remain unclear. Therefore, it is imperative to elucidate the underlying mechanism governing the induction of HSP40 expression during heat stress and explore its potential as a promising therapeutic strategy against tumor development. By strategically modifying the aza-BODIPY structure to precisely distribute the excited-state energy, we have demonstrated that HSP40 specific expression is correlated with the proportion of heat to hydroxyl radicals rather than their individual levels. This orchestrated NIR-II photosensitizer-based nanoparticles reduced tumor glycolysis and disrupted ATP production, driving cell apoptosis and amplifying the efficacy of photothermal therapy. Silencing and compensation of HSPs under heat and ROS stress represent a promising and effective strategy for overcoming tumor self-defense mechanisms in cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


