传递包裹:激活免疫调节的紫贻贝细胞间纳米塑料转移
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
纳米塑料(NPs)通常被认为具有明确的细胞内命运,由于其稳定性而难以排泄或运输。本研究提供了绿贻贝(Perna viridis)血细胞中NPs细胞间转移的第一个证据,该转移随后激活了免疫调节过程。NPs主要被粒细胞内化,一部分被易位并沉积在溶酶体中,而保留在核内体中的NPs随后被转移到新的血细胞(主要是粒细胞)。转移方向受胞内NP浓度梯度驱动。转移动力学依赖于大小,较小的NPs表现出更大的潜力,但速率较低,主要是由于它们具有特定的细胞外囊泡介导的转移途径。隧道纳米管为NPs的细胞间转移提供了最有效的途径,因为其连续的膜结构允许直接的物质交换。至关重要的是,NP再分配伴随着线粒体向受损血细胞的梯度驱动转移。这一过程减轻了负担过重的血细胞的应激,调节了活性氧的产生,从而增强了吞噬活性,促进了免疫反应。这些发现强调NPs在免疫系统中表现出比以前理解的更活跃的行为,并为免疫细胞如何在面对NP挑战时维持海洋生物的健康提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
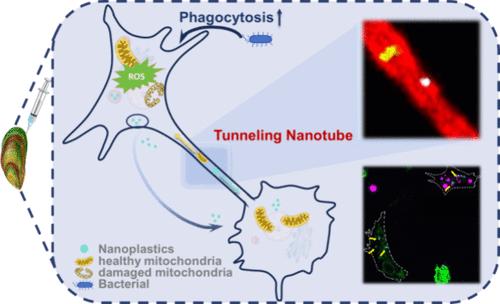
Passing the Parcels: Intercellular Nanoplastics Transfer in Mussels Perna viridis with Activated Immunomodulation
Nanoplastics (NPs) are generally considered to have a defined intracellular fate, being difficult to excrete or transport due to their stability. This study provides the first evidence of NPs intercellular transfer in the hemocytes of green mussels (Perna viridis), which subsequently activated the immunomodulation process. NPs were predominantly internalized by granulocytes, with a portion being translocated and deposited in lysosomes, whereas those retained in endosomes were subsequently transferred to new hemocytes (mainly granulocytes). The transfer direction was driven by the intracellular NP concentration gradients. Transfer kinetics was size-dependent, with smaller-sized NPs exhibiting greater potential but a lower rate, primarily due to their specific extracellular vesicle-mediated transfer pathway. Tunneling nanotubes provided the most efficient pathway for the intercellular transfer of NPs, as their continuous membrane structure allowed direct substance exchange. Crucially, NP redistribution was accompanied by a gradient-driven transfer of mitochondria to injured hemocytes. This process alleviated stress on the overburdened hemocytes and regulated reactive oxygen species production, subsequently enhancing phagocytic activity and promoting immune responses. These findings underscore that NPs exhibit far more active behavior in the immune system than previously understood and provide new insights into how immune cells maintain the health of marine organisms in the face of NP challenges.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


